Pharmaceutical composition containing sildenafil citrate, and preparation method and application thereof
A technology for sildenafil citrate and its composition, which is applied in the field of pharmaceutical compositions containing sildenafil citrate, and can solve problems such as high cost, difficulty in taking divided doses for patients, and easy hygroscopicity of finished products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
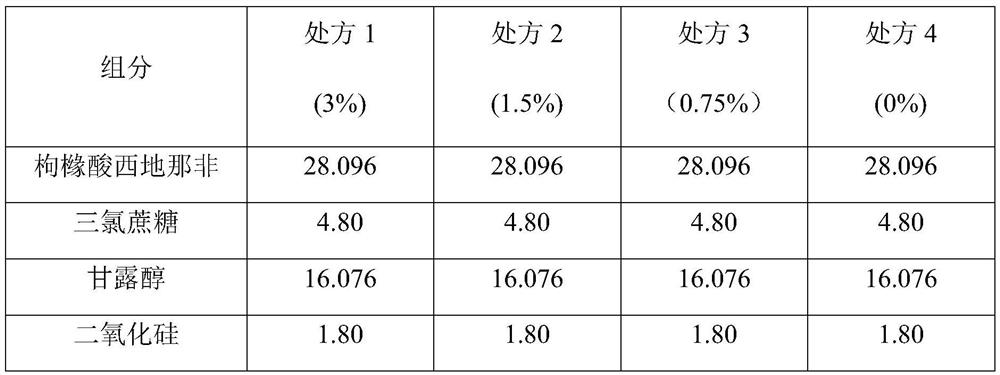
[0078] Embodiment 1 (10mg / tablet)
[0079]
[0080] Preparation:
[0081] 1. Sildenafil citrate, povidone k30, mannitol and colloidal silicon dioxide are passed through a 600um sieve and added to a wet mixing granulator for premixing.
[0082] 2. After premixing, add water to granulate in the wet mixing granulator, and use the 8000μm sieve for wet granulation of the prepared soft material.
[0083] 3. Drying: Fluidized bed drying is used to control the moisture content of the material to ≤3%.
[0084] 4. Carry out dry sizing of the dried dry granules with a sieve aperture of 1000 μm.
[0085] 5. Add colloidal silicon dioxide, sucralose, crospovidone, croscarmellose sodium, and microcrystalline cellulose to the dry granulated material, and mix evenly with a mixer.
[0086] 6. Add the material to magnesium stearate and mix evenly.
[0087] 7. Tablet pressing.
[0088] 8. Packaging.
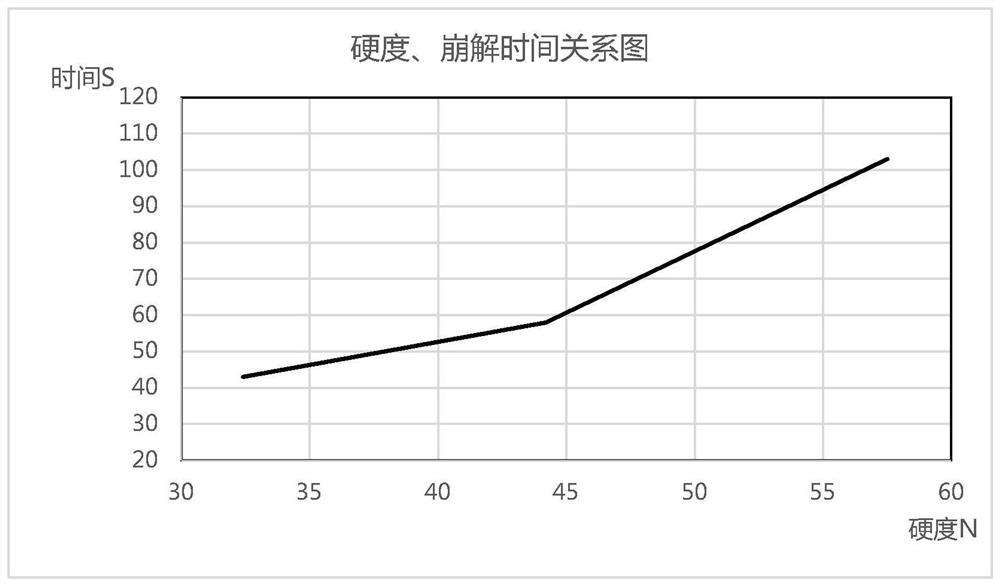
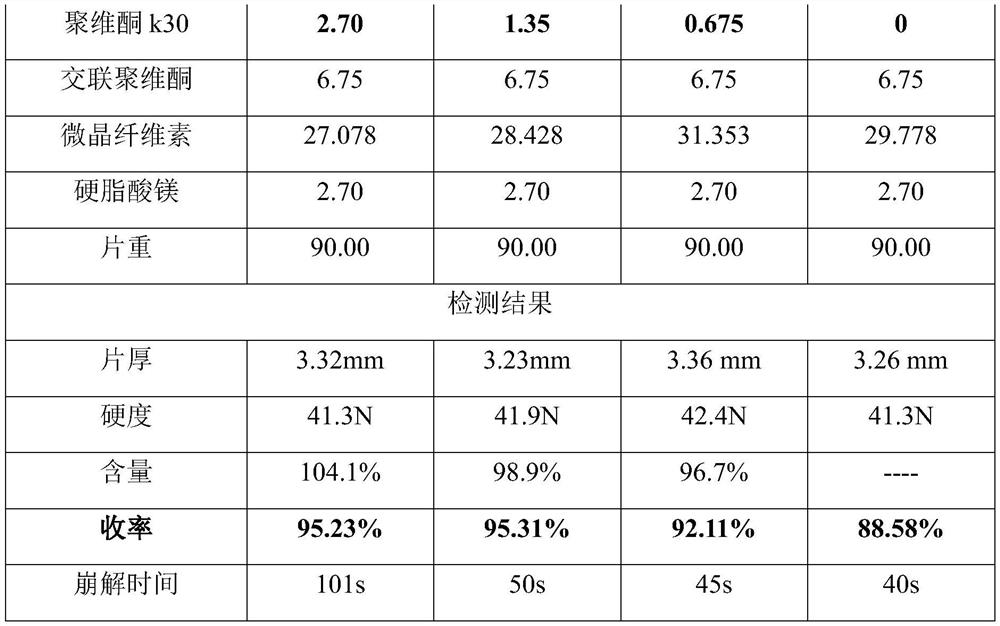
[0089] Tablets with different hardness were pressed at different speeds on the rotary t...
Embodiment 2
[0098] Embodiment 2 (5mg / tablet)
[0099]
[0100] Preparation:
[0101] 1. Sildenafil citrate, povidone k15, mannitol and colloidal silicon dioxide are passed through an 850um sieve and added to a wet mixing granulator for premixing.
[0102] 2. After premixing, add water to granulate in a wet mixing granulator, and use a 6000μm sieve for wet granulation of the prepared soft material.
[0103] 3. Drying: Fluidized bed drying is used to control the moisture content of the material to ≤3%.
[0104] 4. Carry out dry sizing of the dried dry granules, and the sieve aperture is 1200 μm.
[0105] 5. Add colloidal silicon dioxide, essence, crospovidone, croscarmellose sodium, and microcrystalline cellulose to the dry granulated material, and mix evenly with a mixer.
[0106] 6. Add the material to magnesium stearate and mix evenly.
[0107] 7. Tablet pressing, the tablet range is 25-55N.
[0108] 8. Packaging.
Embodiment 3
[0109] Embodiment 3 (20mg / tablet)
[0110]
[0111]
[0112] This embodiment is made on the basis of embodiment 1, increased in equal proportions, and the specification is: 20mg / tablet (based on sildenafil). The preparation method is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com