Dispersible tablet containing amoxicillin and potassium clavulanate
A technology of potassium clavulanate and amoxicillin, which is applied in the field of dispersible tablets containing amoxicillin and potassium clavulanate, can solve the problems of heavy economic burden for patients, low dissolution rate and dissolution rate, and inconvenient administration, and achieve Excellent dispersion uniformity and dissolution rate, reduced medication burden, and shortened time to peak
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
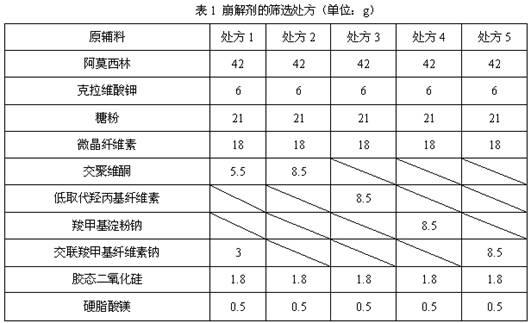
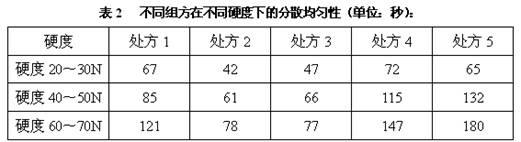
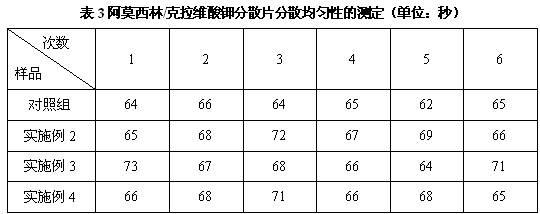
[0052] Example 1 Screening study of disintegrants
[0053]The dispersible tablets of the prior art that contain amoxicillin and potassium clavulanate mostly adopt the mixed disintegrant of croscarmellose sodium and crospovidone, but because crospovidone, croscarmellose Plain sodium is more expensive, and the use of disintegrants increases production costs. On the premise of ensuring the quality of the finished product, in order to reduce the production cost, the inventors further screened many disintegrants. Specifically, in terms of the selection of disintegrants, the inventors have found through a large number of experiments: the use of sodium carboxymethylcellulose, precrossified starch, and calcium sodium carboxymethylcellulose as a single disintegrant cannot meet the technical requirements of the present invention. Therefore, cross-linked sodium carboxymethyl cellulose, sodium carboxymethyl starch, low-substituted hydroxypropyl cellulose and cross-linked povidone were ...
Embodiment 2
[0057] Example 2 Preparation of Amoxicillin / Potassium Clavulanate Dispersible Tablets
[0058] Prescription composition:
[0059] Amoxicillin 420 g;
[0060] Potassium clavulanate 60 g;
[0061] 210 g powdered sugar;
[0062] Microcrystalline cellulose 180 g;
[0063] Low-substituted hydroxypropyl cellulose 85 g;
[0064] Colloidal silicon dioxide 18 g;
[0065] Magnesium stearate 5 g.
[0066] Preparation Process:
[0067] Under the conditions of temperature ≤ 20°C and relative humidity ≤ 20%, follow the steps below:
[0068] (1) Weigh the raw and auxiliary materials of the above prescription quantity, and the prescription quantity of amoxicillin-clavulanate potassium mixed powder is calculated as clavulanic acid.
[0069] (2) Dry the raw and auxiliary materials except amoxicillin-clavulanate potassium mixed powder and colloidal silicon dioxide, in which the temperature of amoxicillin and magnesium stearate is set at 40±5°C for 5 hours. The rest of the accesso...
Embodiment 3
[0074] Example 3 Preparation of Amoxicillin / Potassium Clavulanate Dispersible Tablets
[0075] Prescription composition:
[0076] Amoxicillin 420 g;
[0077] Potassium clavulanate 60 g;
[0078] 160 g powdered sugar;
[0079] Starch 190 g;
[0080] Low-substituted hydroxypropyl cellulose 100 g;
[0081] Colloidal silicon dioxide 20 g;
[0082] Magnesium stearate 5 g.
[0083] Preparation process: with embodiment 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com