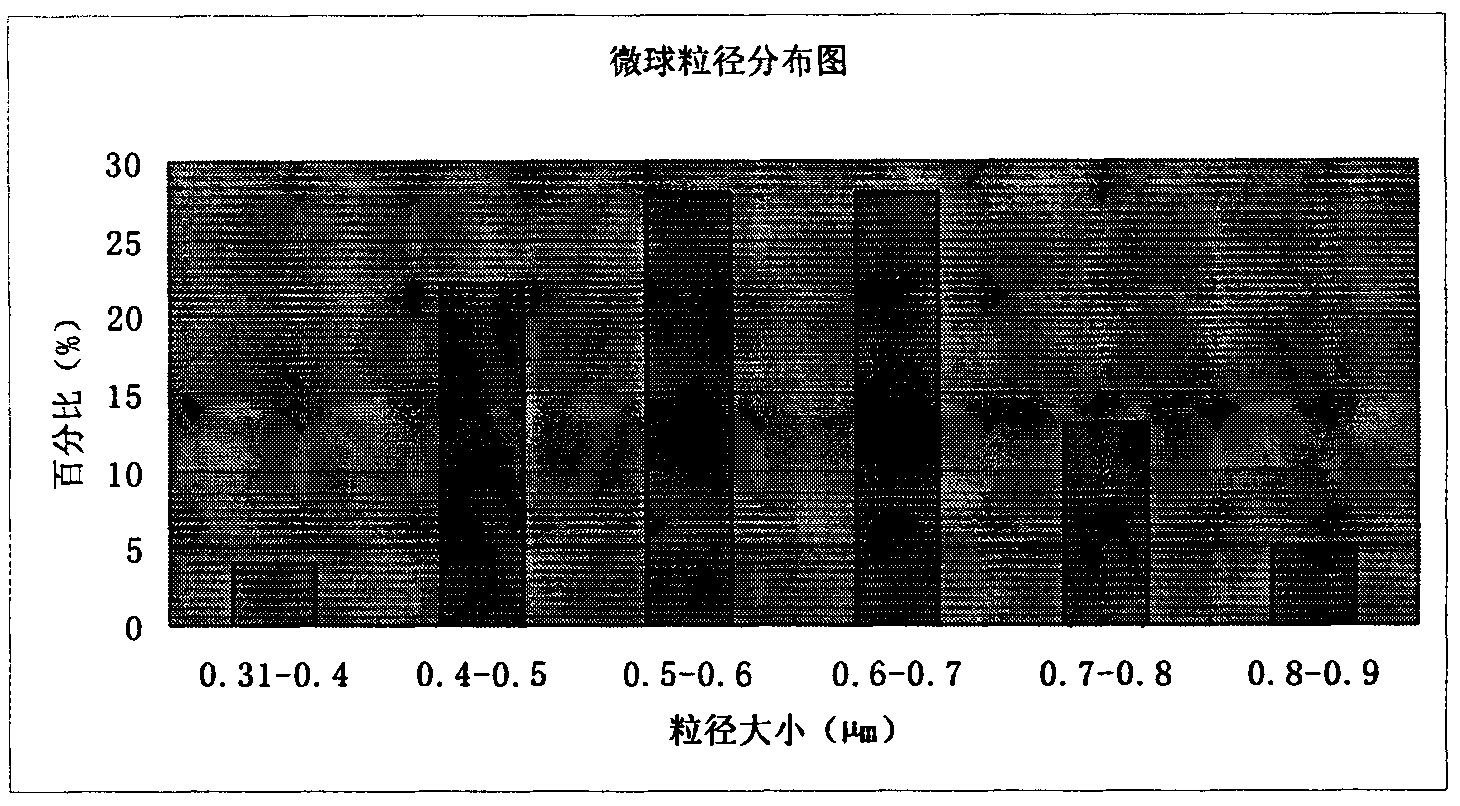
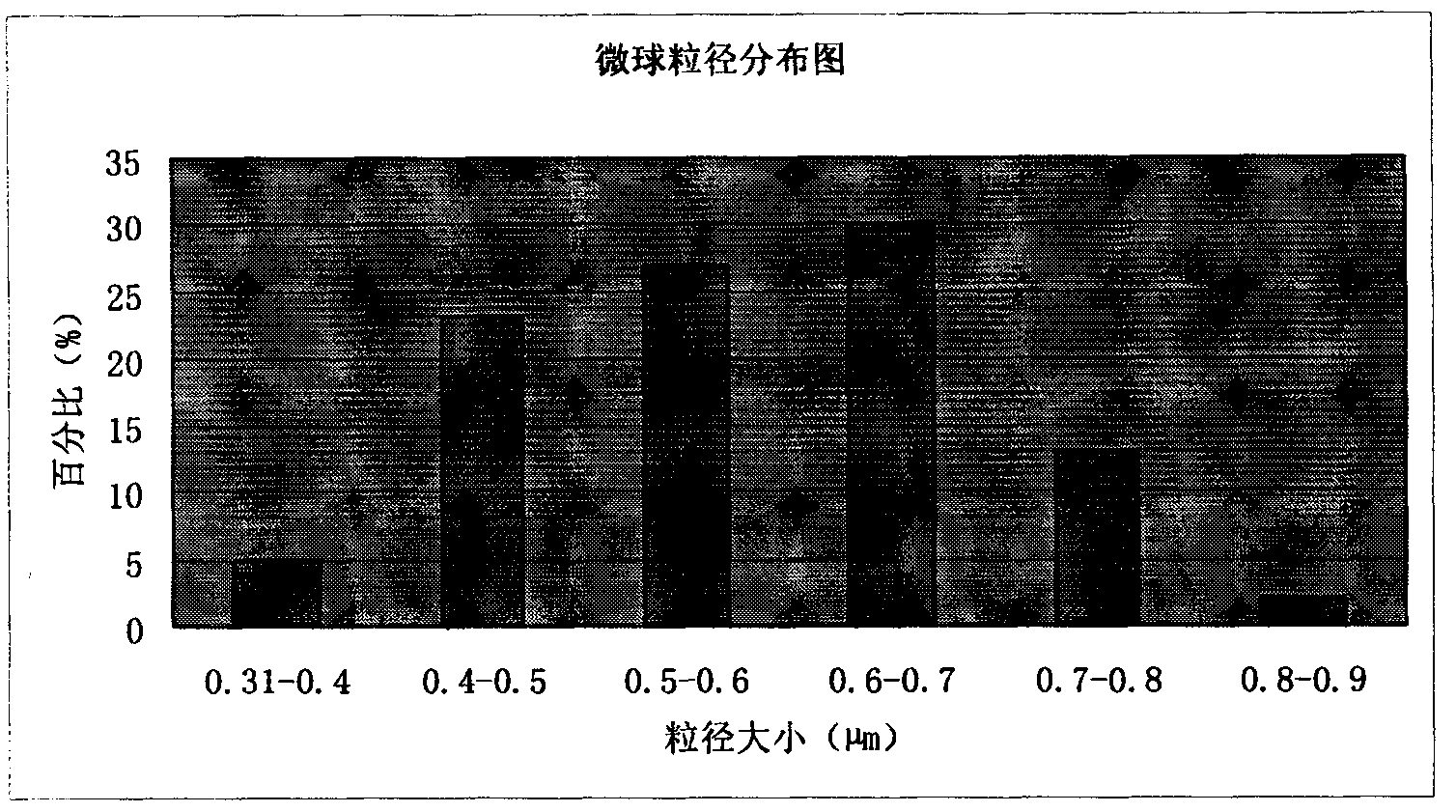
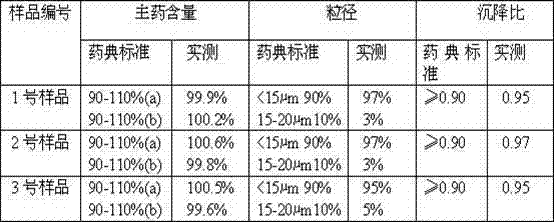
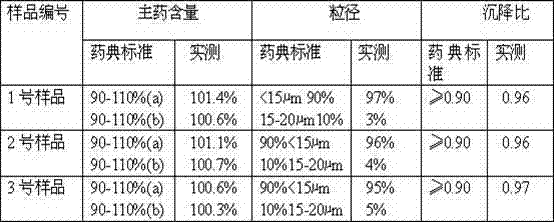
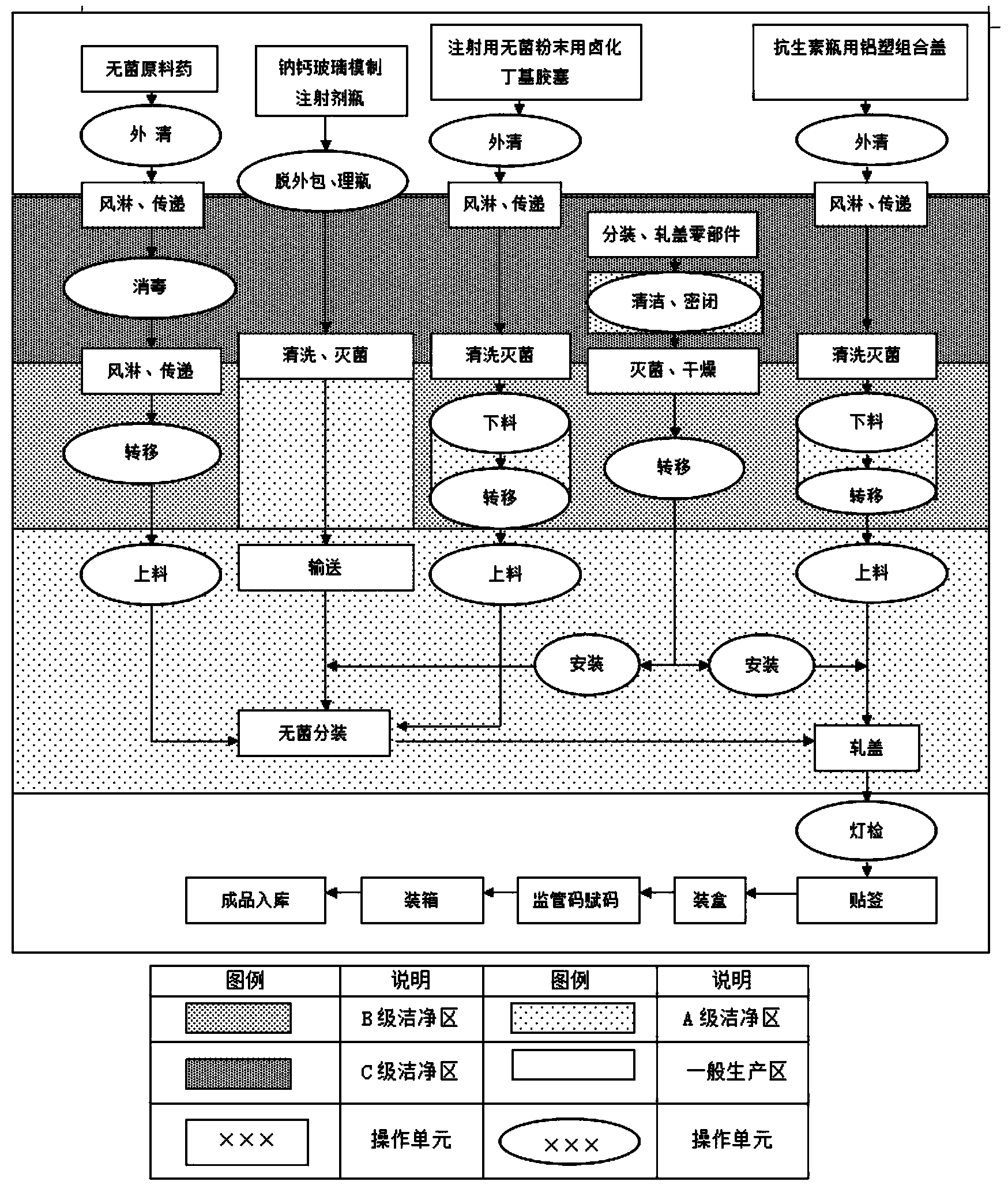
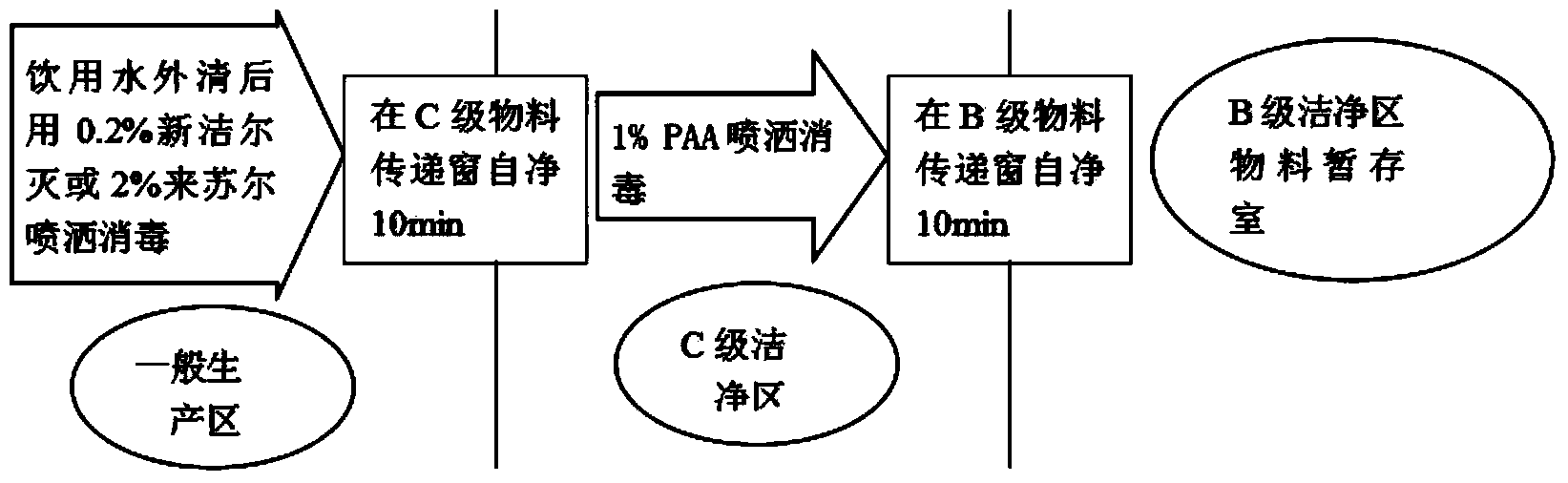
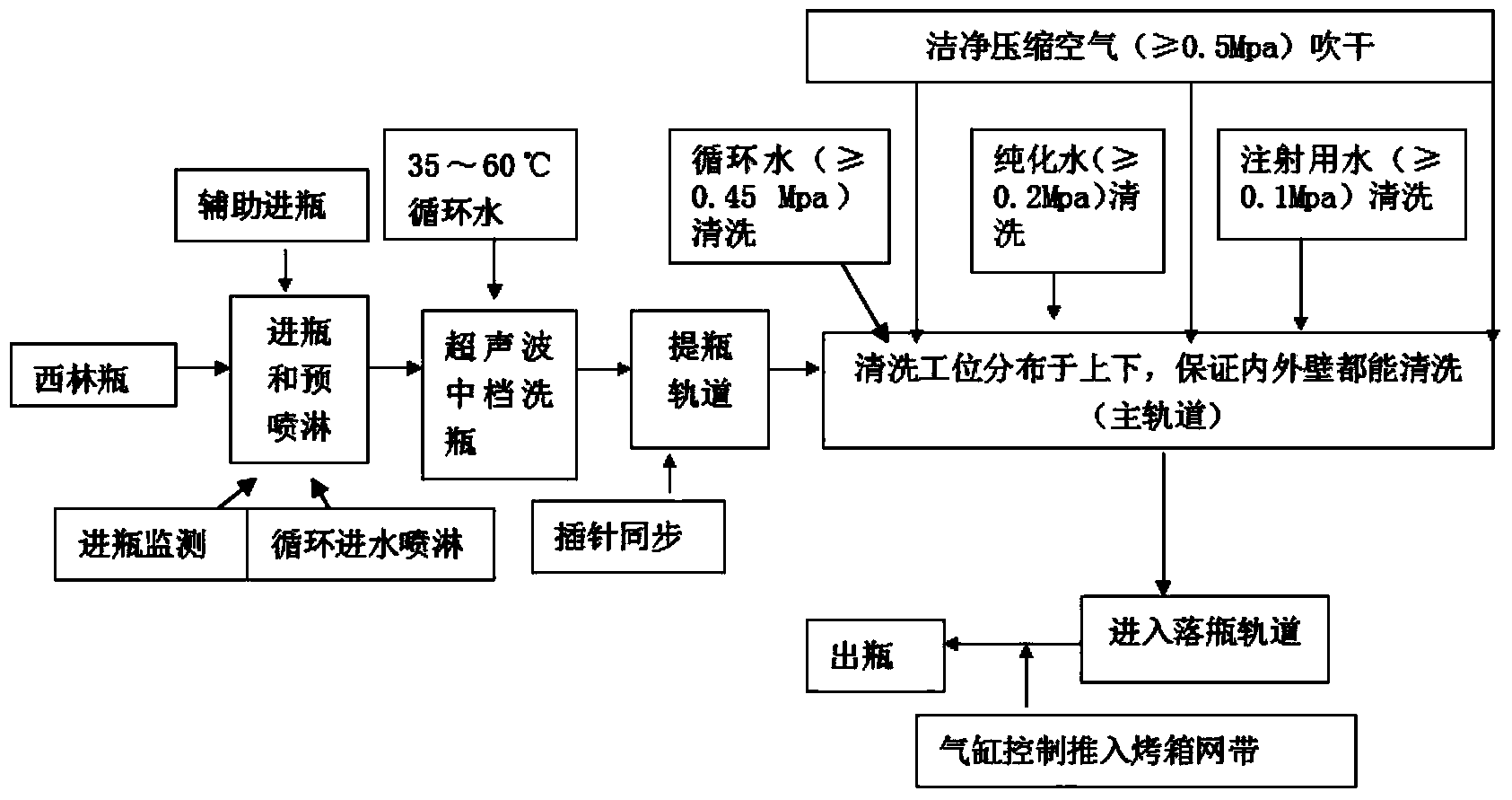
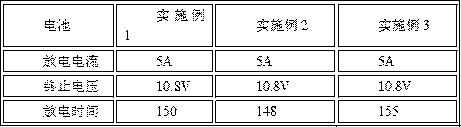
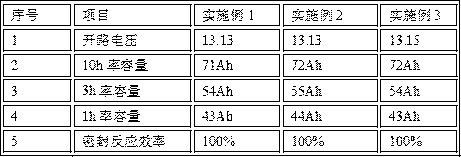
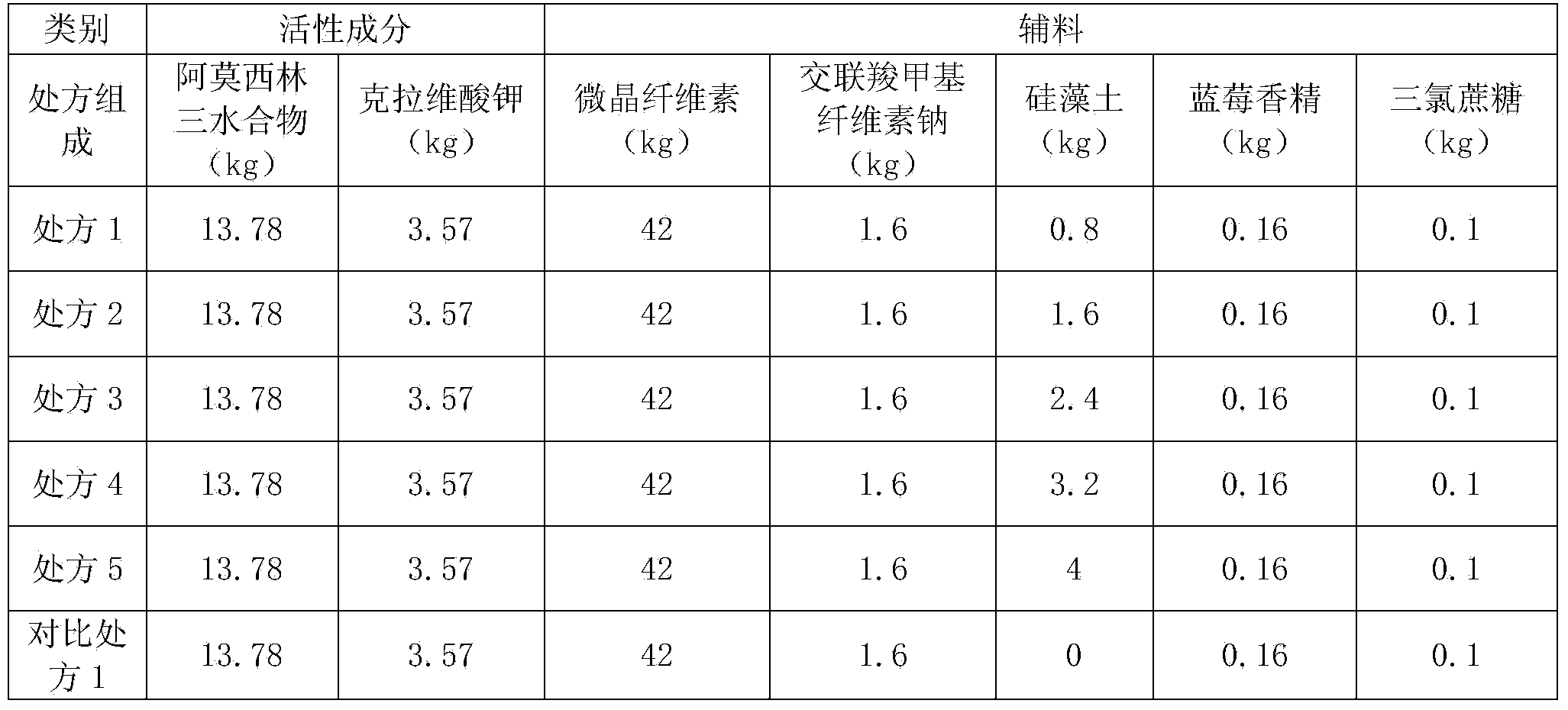
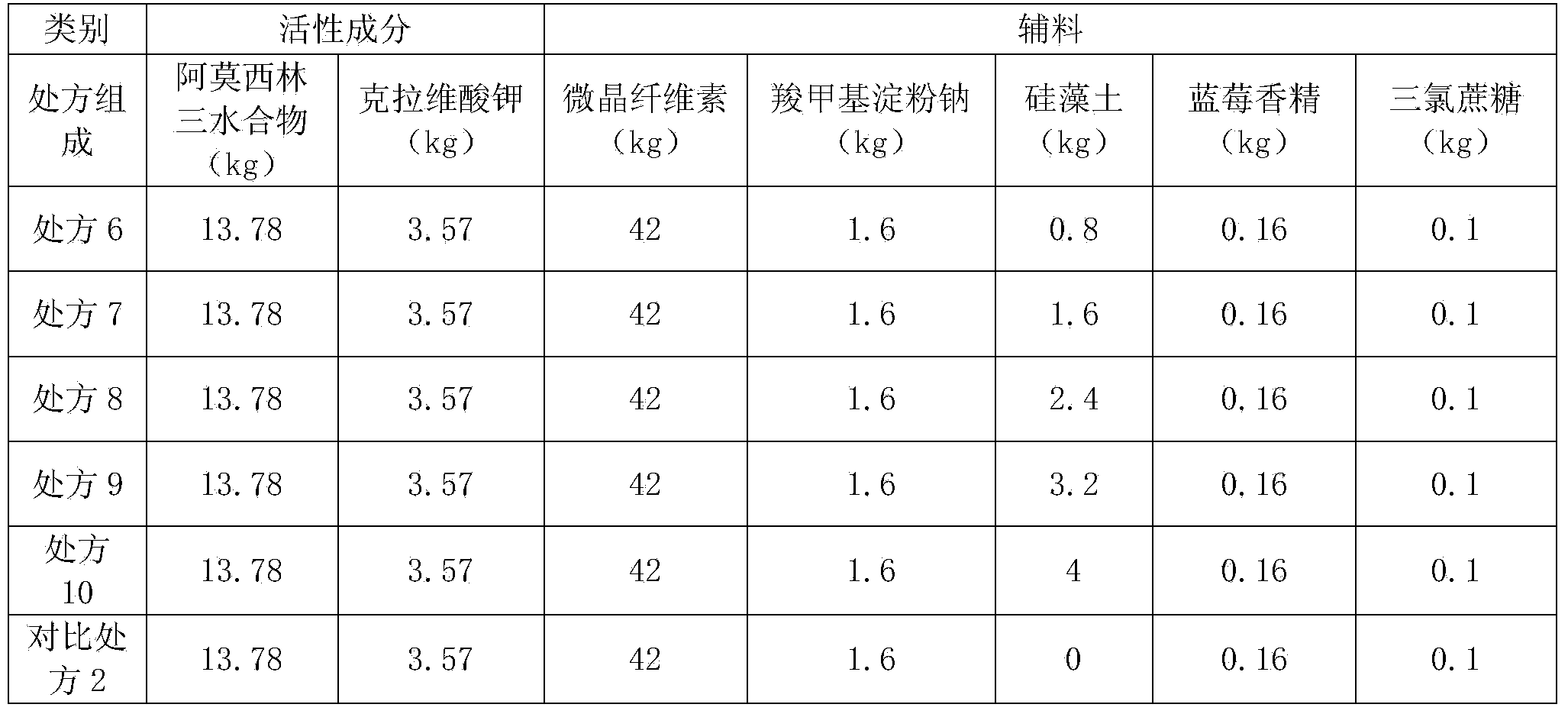
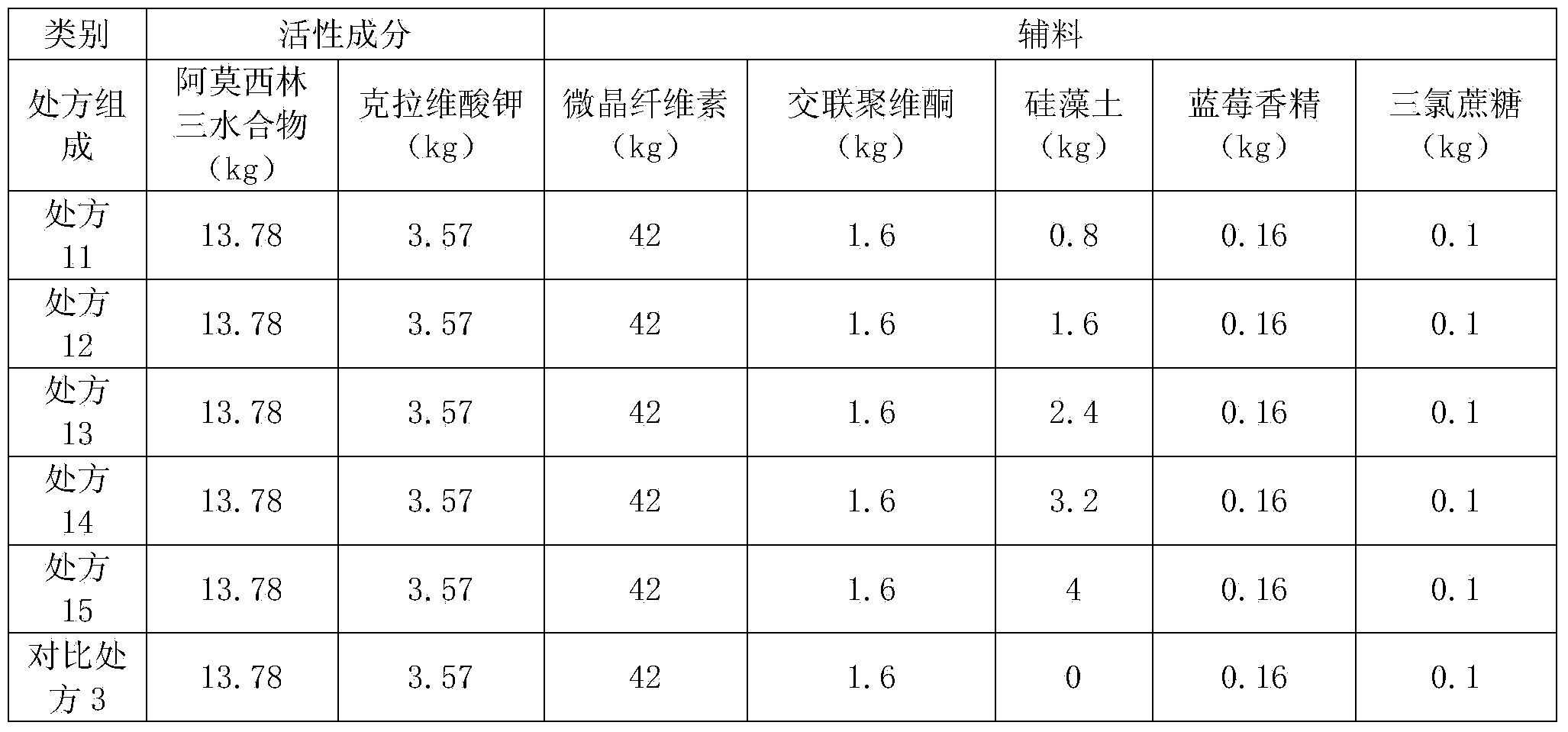
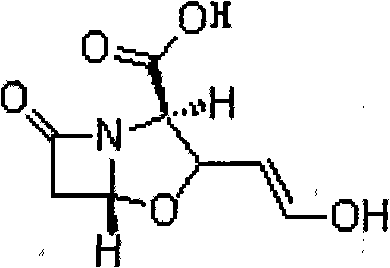
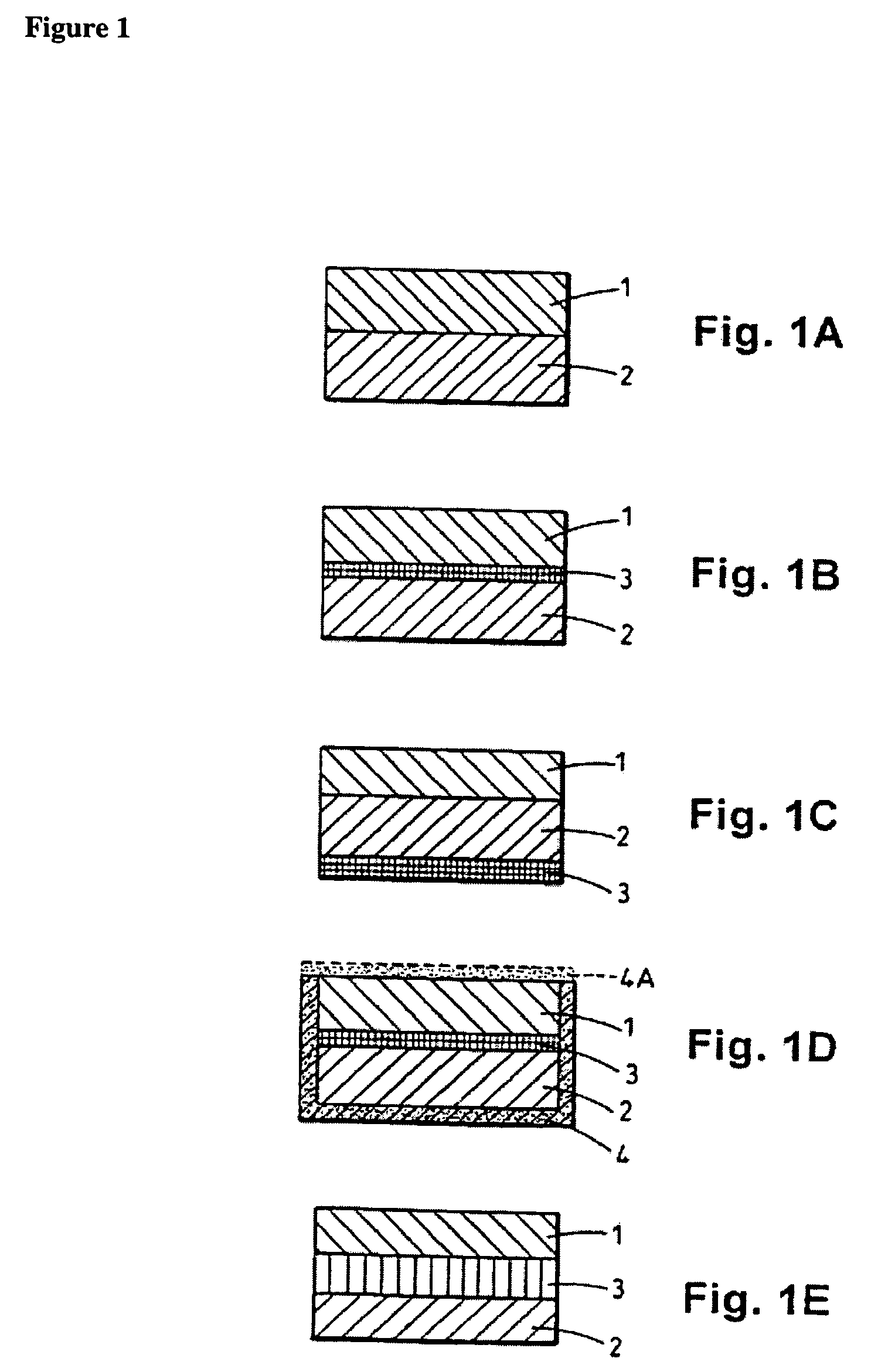
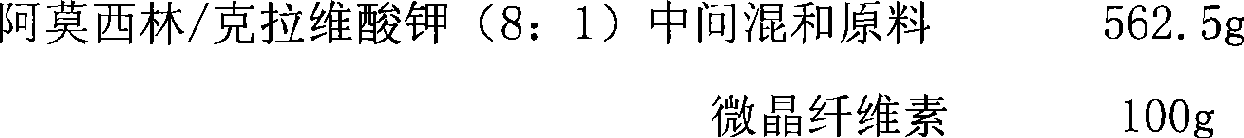Patents
Literature
112 results about "CLAVULANATE POTASSIUM" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Amoxicillin/clavulanate potassium tablet and preparation method thereof
InactiveCN101502511AEasy to operateReduce manufacturing costAntibacterial agentsPill deliveryAmoxicillin-clavulanate potassiumCombinatorial chemistry
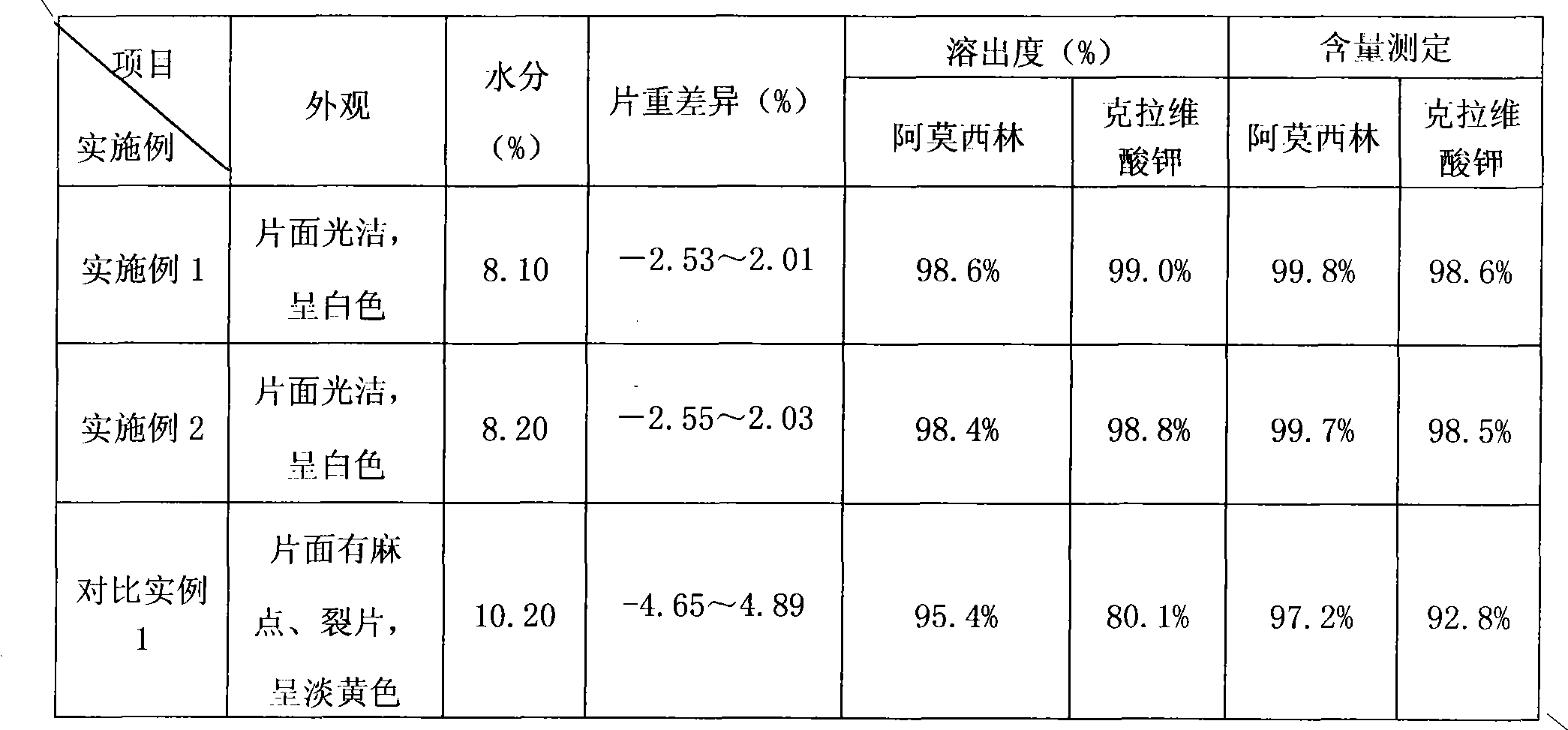
The invention provides an amoxicillin / clavulanate (8:1) tablet and a preparation method thereof. The amoxicillin / clavulanate (8:1) tablet has the advantages of simple preparation process, good quality controllability and stability and low forming-production cost by adopting the mixed amoxicillin / clavulanate (8:1) intermediate materials having good quality controllability and stability as the main drug resource.
Owner:山东淄博新达制药有限公司
Preparation method of amoxicillin and clavulanate potassium tablets
InactiveCN101897701AReduce exposure timeGuaranteed stabilityAntibacterial agentsPharmaceutical product form changeMass ratioDissolution
The invention discloses a preparation method of amoxicillin and clavulanate potassium tablets, comprising the following steps: (a) weighing the following main and auxiliary materials: 120-135 parts of amoxicillin, 40-45 parts of clavulanate potassium, 27-32 parts of microcrystalline cellulose, 1-3 parts of croscarmellose sodium (ADS), 1-3 parts of superfine silica powder and 2-4 parts of magnesium stearate; (b) after pelletizing amoxicillin, mixing the pelletized amoxicillin with clavulanate potassium according to the mass ratio of 4:1 to form the main materials; (c) uniformly mixing the auxiliary materials including superfine silica powder, ADS and microcrystalline cellulose by the method of increment by equal quantity and throwing the main materials and the auxiliary materials into a mixer by the method of increment by equal quantity to be mixed for 36-70min; and (d) tabletting the mixed powder. The products prepared by the method have bright, clean and beautiful appearances and stable content and dissolution.
Owner:NORTH CHINA PHARMA COMPANY
Beta-cyclodextrin / amoxicillin inclusion compound and its composition with clavulanic kalium and preparation thereof
InactiveCN1698604AImprove drug stabilityPromote dissolutionAntibacterial agentsHeterocyclic compound active ingredientsCellulosePolyethylene glycol
The invention provides a beta-cyclodextrin / amoxicillin inclusion compound medicinal composition which comprises amoxicillin and beta cyclodextrin by the weight ratio of 1:2.5-1:5. In a preferred embodiment, the composition also comprises clavulanate potassium, wherein the mass ratio of amoxicillin and clavulanate potassium is 14:1-2:1, the supplementary materials are selected from crystalline cellulose, maize starch, citric acid, talcum powder, sodium carboxymethylstarch, stearic acid, calcium stearate, magnesium stearate, amylopectin, lactose, mannitol, crosslinked povidone, talcum powder, polyethylene glycol 4000, and low substituted methylcellulose propylene glycol ether. The invention also discloses the preparing process.
Owner:NANJING J ONE MEDICAL TECH DEV
Beta- lactamase suppressing antibacterial compound drugs
InactiveCN1565457AHigh tissue contentWide distribution in the bodyAntibacterial agentsOrganic active ingredientsCompounding drugsCeftizoxime
The invention discloses a beta- lactamase suppressing antibacterial compound drugs, which comprises ceftizoxime, or cefodizime and beta-lactam enzyme inhibitor by the active acid weight ratio of 1-10:10-1, which are in the forms of alkali metal salts or free acid and assisting solvents, the beta-lactam enzyme inhibitor can be Tazobactam, or clavulanic acid, or tapazole or their derivatives.
Owner:张哲峰
Novel detection method of injection use compound amoxicillin sodium and clavulanate potassium
InactiveCN101776675AComponent separationAnalysis by thermal excitationAdditive ingredientAmoxicillin Sodium
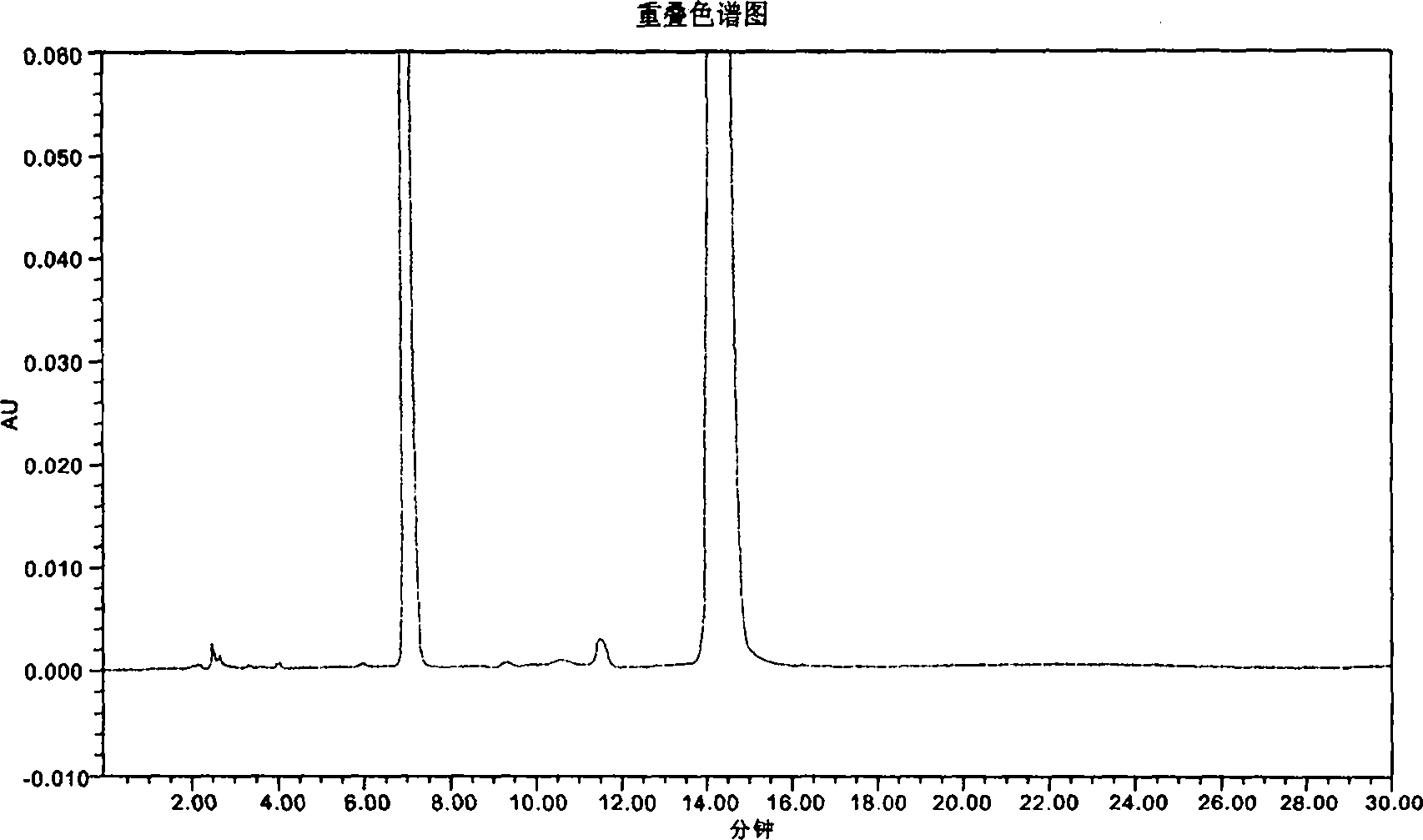
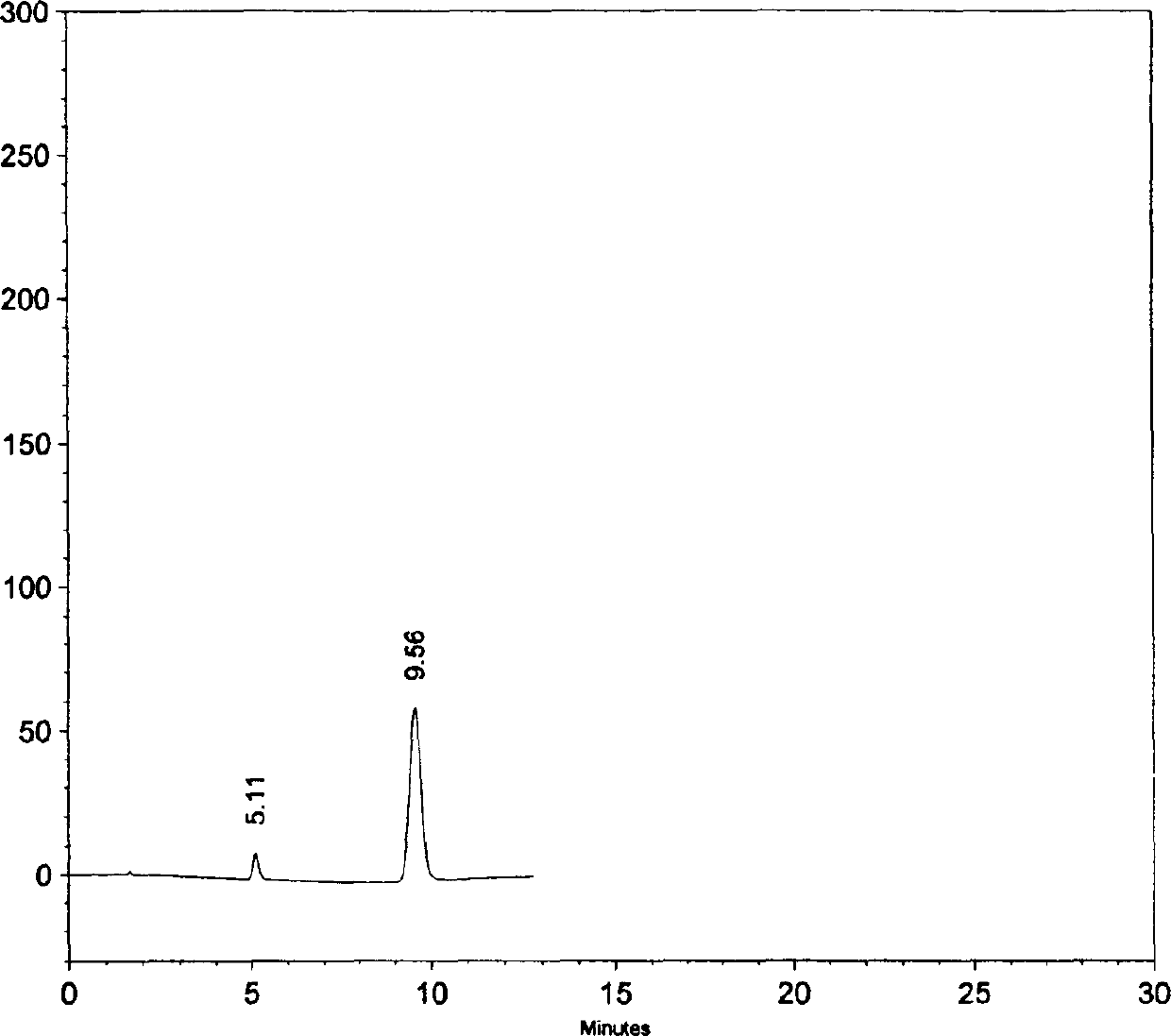
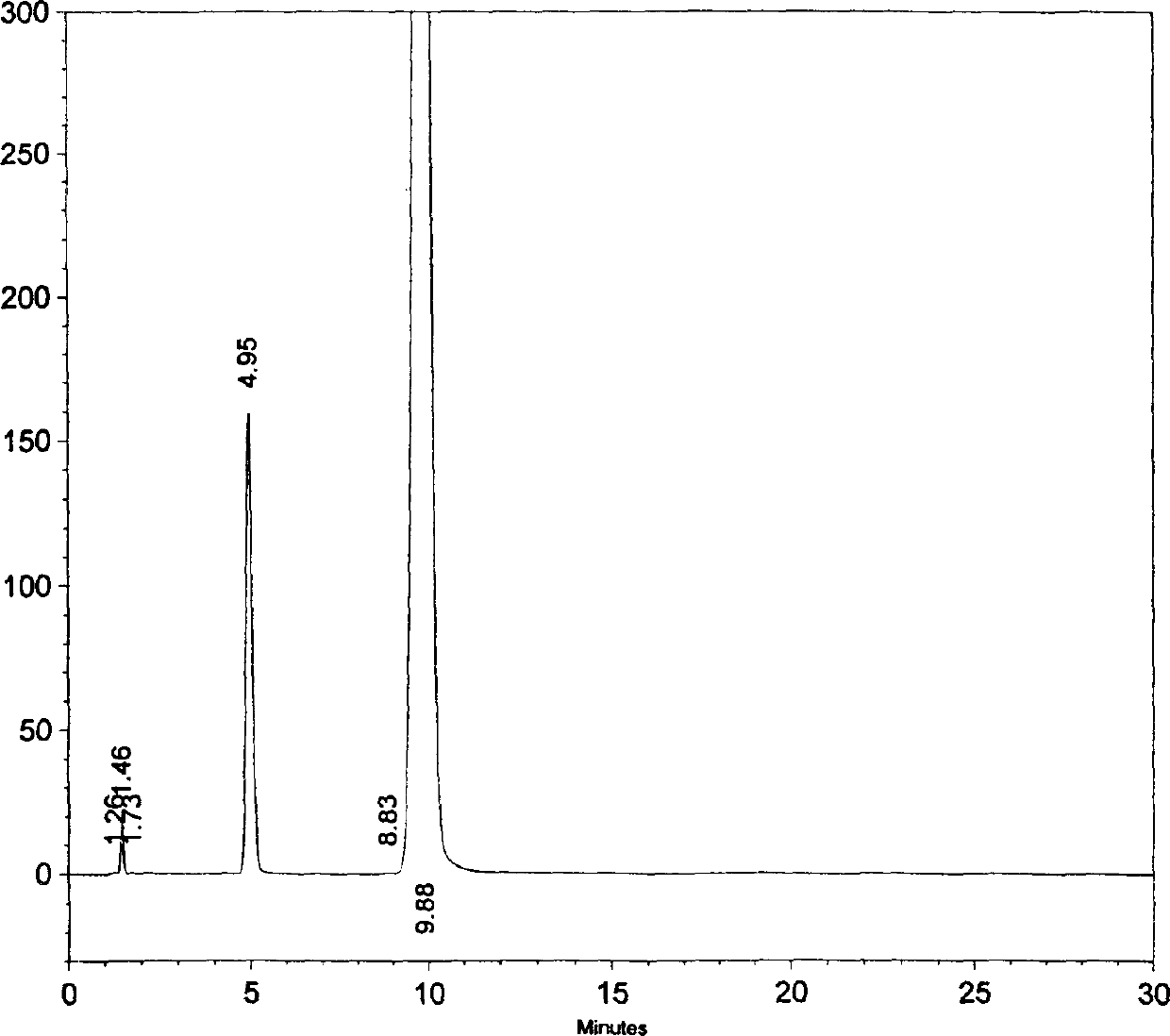
The invention provides a novel high-efficiency liquid chromatography (HPLC) method capable of synchronously detecting the contents of two single ingredients and relevant impurities in injection use compound amoxicillin sodium and clavulanate potassium. Compared with the existing detection method, the invention has higher precision on the 7 / 1 compound preparation with relatively low clavulanic acid content, and has better separation degree on the injection preparation with higher purity requirement, in addition, the two ingredients of the amoxicillin and the clavulanic acid can not be mutually interfered or influenced in the detection process. The method has the advantages of simple operation, easy implementation, strong specificity, high sensitivity, large linear range, good stability and good repetitiveness, and can be used for detecting compound sterile injection preparations and raw materials.
Owner:XIANGBEI WELMAN PHARMA CO LTD
Liposome injection of pharmaceutical composition of amoxicillin sodium and clavulanate potassium
InactiveCN101804051AImprove stabilityHigh encapsulation efficiencyAntibacterial agentsPharmaceutical non-active ingredientsFreeze-dryingAntioxidant
The invention provides a liposome injection of a pharmaceutical composition of amoxicillin sodium and clavulanate potassium, which consists of amoxicillin sodium, clavulanate potassium, a liposome carrier, a lyoprotectant and a freely optional antioxidant, wherein the liposome carrier is egg yolk phosphatidylinositol and sodium taurocholate. The liposome injection has good preparation stability, liposome can avoid the fracture due to dehydration, fusion, ice crystal generation and the like during the freeze-drying process, and the liposome can still keep the good encapsulation rate after hydration and redissolution.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Amoxicillin and clavulanate potassium injection and preparation method thereof
The invention belongs to the technical field of medical technology, and particularly relates to an amoxicillin and clavulanate potassium injection and a preparation method of the amoxicillin and clavulanate potassium injection. The amoxicillin and clavulanate potassium injection includes amoxicillin sodium, potassium clavulanate and a pH regulator. The preparation method comprises the steps of crushing and mixing the amoxicillin sodium, the potassium clavulanate and the pH regulator uniformly through air flows according to certain proportion, then respectively packaging the mixture into penicillin bottles, controlling the water content to be 0.5%, filling nitrogen for protection, plugging, rolling a cover and finally packaging. According to the invention, the obtained product is stable in quality, the problems of the color changing and the deterioration caused by the easy oxidization of the similar products can be solved, the product quality is improved, and high economical benefits are obtained.
Owner:LUNAN BETTER PHARMA
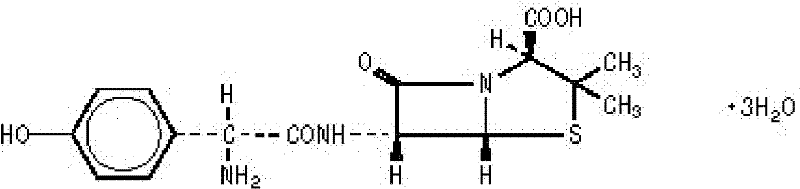
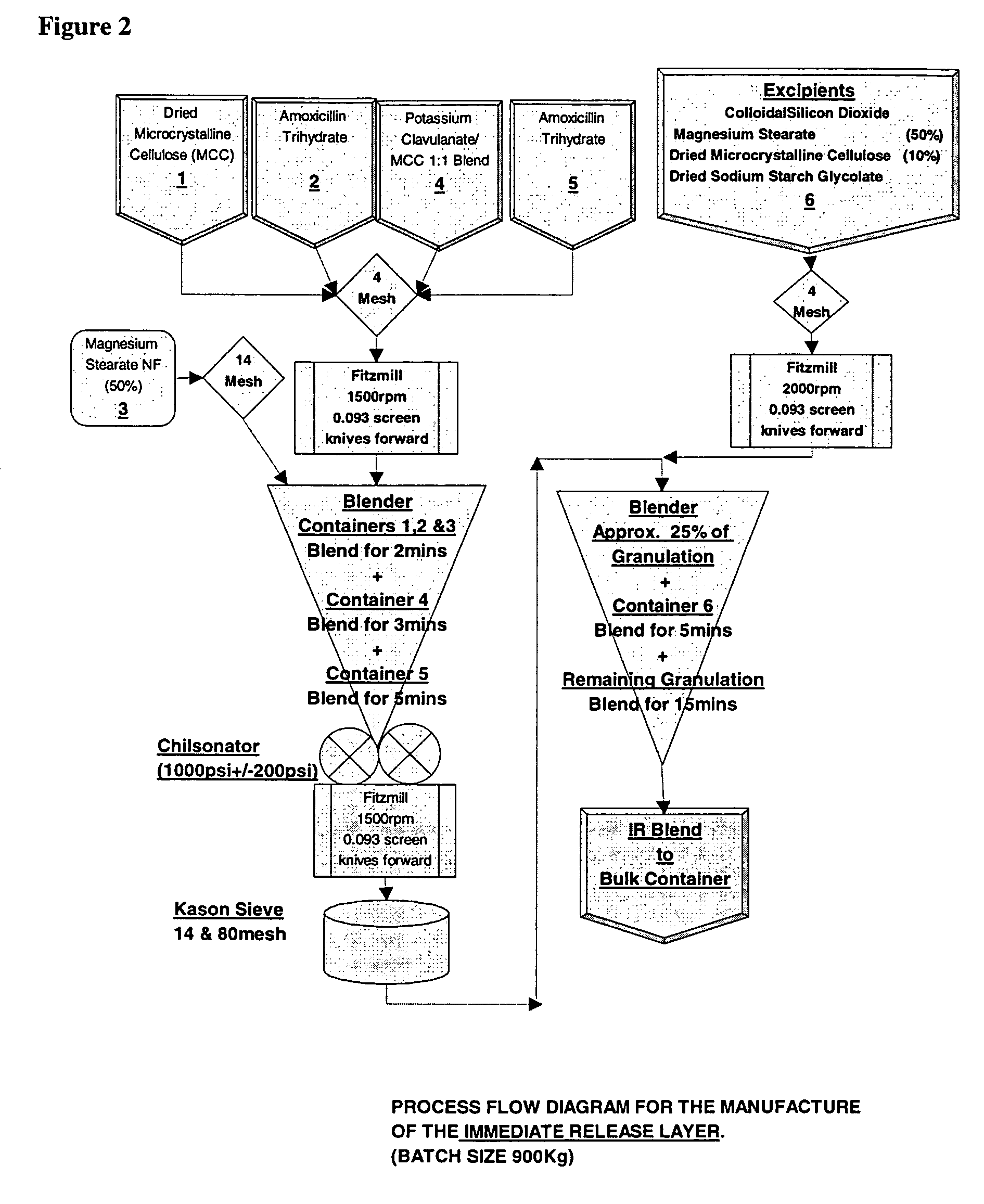
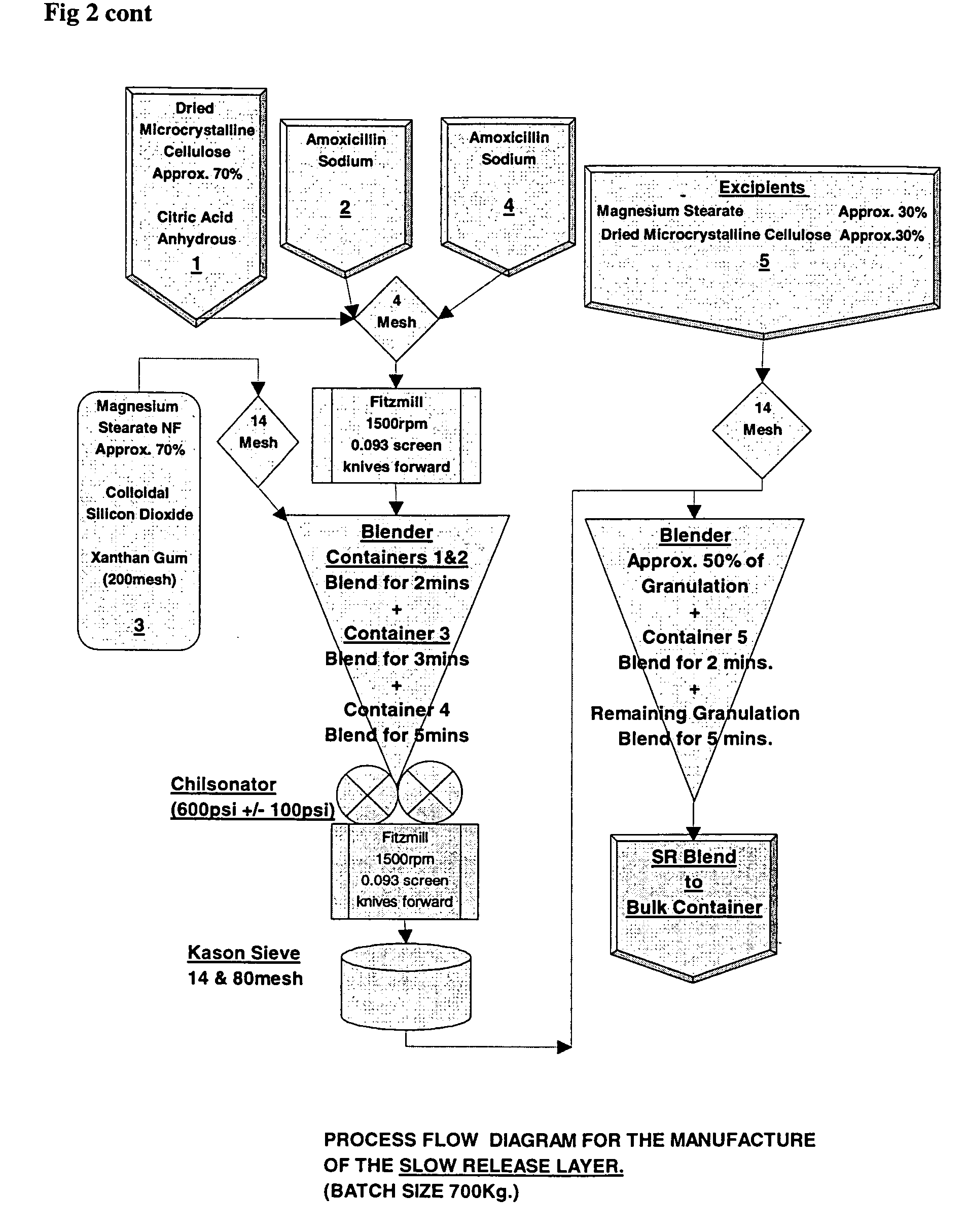
Pharmaceutical composition containing amoxicillin trihydrate and clavulanate potassium
InactiveCN1634044ALow priceRaw materials are easy to getAntibacterial agentsPharmaceutical delivery mechanismAdjuvantMethyl cellulose
The invention discloses a slow release medicament three-layer tablet which comprises Amoxicillin hydrate, clavulanate potassium and pharmaceutically acceptable excipient or carrier, the layer 1 is a rapid release layer comprising Amoxicillin hydrate and clavulanate potassium, the layer 2 is a rapid release layer comprising a portion of Amoxicillin hydrate, the layer 3 is a rapid release layer comprising rest portions of Amoxicillin hydrate, the slow release layer contains not only common medicinal adjuvant, but also slow release material of hydroxy propyl methyl cellulose E30.
Owner:北京泛美华医药科技发展有限公司
Amoxicillin or amoxicillin and clavulanate potassium instant chewable tablets for pet and preparation method for same
InactiveCN102512386AGive full play to the antibacterial effectSolve the convenienceAntibacterial agentsPill deliveryMedicineOrally disintegrating tablet
The invention belongs to the field of veterinary medicine, and particularly relates to amoxicillin or amoxicillin and clavulanate potassium instant chewable tablets for pet and a preparation method for the same. The instant chewable tablets are the amoxicillin or amoxicillin and clavulanate potassium instant chewable tablets prepared by combining with orally disintegrating tablets fast in medicine release speed in oral cavity and bad in disintegrating crispness and chewable tablets chewable but slow in disintegrating and medicine release speeds on the basis of the present dosage form of amoxicillin or amoxicillin and clavulanate potassium, so that administration for pet is greatly facilitated, and the antibacterial characteristic of amoxicillin can be adequately exerted. The preparation method for the instant chewable tablets comprises the following steps of: crushing amoxicillin or amoxicillin and clavulanate potassium and related auxiliary materials, and passing through a sieve of 50-100 meshes at first; uniformly mixing the main medicine and the selected auxiliary materials in a proper ratio, and producing wet granules; and drying and straightening the granules, and then tabletting. The instant chewable tablets have broad-spectrum antimicrobial activity and can be used for preventing and treating the bacterial infection of pet. While being used, the preparation is placed in the mouth of pet and fast melted by chewing, so that the convenience and compliance of administration for pet are solved well.
Owner:NANJING AGRICULTURAL UNIVERSITY
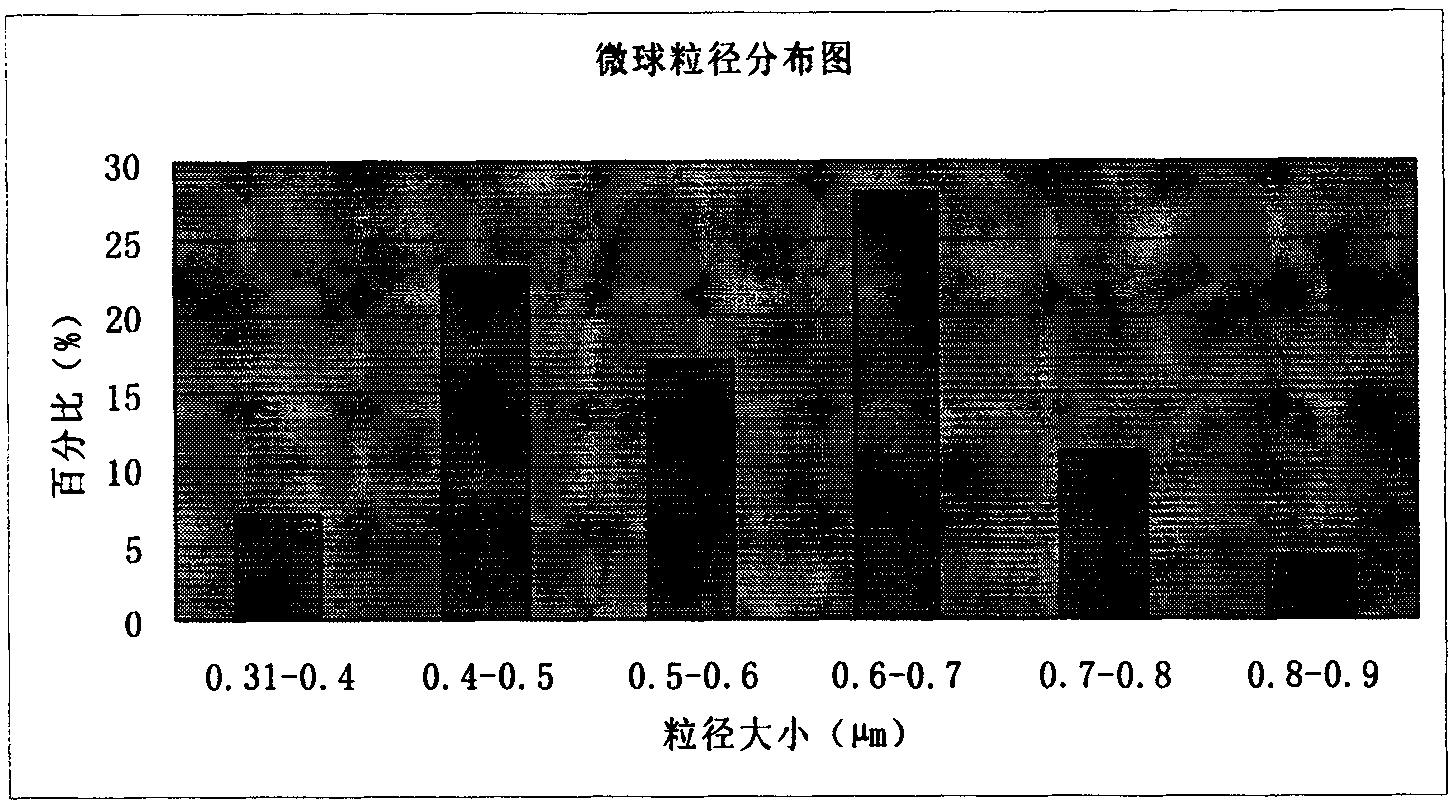
Amoxicillin sodium potassium clavulanate composition microballoon injection
InactiveCN101890007AImprove stabilityHigh encapsulation efficiencyAntibacterial agentsGranular deliveryGlycerolPhysical chemistry
The invention discloses an amoxicillin sodium potassium clavulanate composition microballoon injection, which is characterized by comprising the following components in parts by weight: 5 parts of amoxicillin sodium, 1 part of potassium clavulanate, 4-6 parts of gelatin, 4-6 parts of Arabic gum, 3-6 parts of disodium hydrogen phosphate, 2-4 parts of trehalose and 1-3 parts of glycerol. Compared with the prior art, the amoxicillin sodium potassium clavulanate composition microballoon injection prepared by the invention has good stability, high entrapment rate, good process repeatability, even particle distribution, good injectable property and good slow release character, and is suitable for industrialization production.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Antiseptic composition of mezlocillin
InactiveCN1485035AImprove antibacterial propertiesHigh antibacterial activityAntibacterial agentsHeterocyclic compound active ingredientsSolubilitySemisynthetic penicillin
An antibiotic composition of meloxine, which is composed of meloxine andª‰-lactamase inhibitor at the weight ratio of 1-10í†10-1 based on the amount of active acids. meloxine is in the form of the alkali salt of meloxine or in the form of its free acid and solubility promoter; theª‰-lactamase inhibitor is clavulanic acid or tazobactam or their derivatives. Besides mixing and preparing beforehand,the composition could be prepared by mixing meloxine andª‰-lactamase inhibitor proportionally in clinical applications, then is administered, ormeloxine andª‰-lactamase inhibitor are administered independently. meloxine andª‰-lactamase inhibitor are synergetic, which could solve the problem of meloxine resistance in clinical applications.
Owner:周宇
Method for simultaneously measuring plasma concentration of amoxicillin and clavulanate potassium
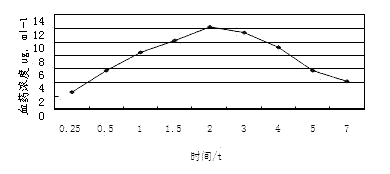
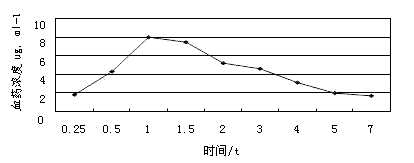
The invention discloses a method for simultaneously measuring the plasma concentration of amoxicillin and clavulanate potassium. The method comprises the steps of depositing proteins of a human plasma sample with methyl alcohol by taking acetaminophen as an interior label, then performing high-speed concentric sample injection, and obtaining the lowest detection concentration of amoxicillin and clavulanate potassium by taking C18 as a chromatographic column and acetonitrile-0.05mmol / L phosphate buffering solution as a flowing phase under the condition that the ultraviolet detection wavelength is 220nm. The method is simple, accurate, sensitive, high in specificity and high in reproducibility and can be used as a plasma concentration measurement method for the biological availability and the bioequivalence study of amoxicillin and clavulanate potassium.
Owner:SICHUAN PHARMA
Amoxicillin/clavulanate potassium enteric-coated preparation composition and preparation method thereof
InactiveCN101890005AWill not affect the curative effectGood controllabilityAntibacterial agentsPharmaceutical product form changeIrritationCurative effect
The invention discloses an amoxicillin / clavulanate potassium enteric-coated preparation composition and a preparation method thereof. The preparation composition is mainly prepared from amoxicillin, clavulanate potassium and an appropriate auxiliary material. Compared with the common amoxicillin / clavulanate potassium preparation, the amoxicillin / clavulanate potassium enteric-coated preparation provided by the invention has the advantages of less irritation to the stomach so as to reduce adverse reaction, and the like. The amoxicillin / clavulanate potassium enteric-coated preparation composition is particularly suitable for patients with stomach upset. The invention provides a novel formulation which has higher safety and better curative effect compared with those of the conventional amoxicillin / clavulanate potassium related preparations; and the preparation process has the advantages of high quality controllability and stability.
Owner:北京瑞伊人科技发展有限公司 +1
Compound amoxicillin and clavulanate potassium tablet and preparation method thereof
ActiveCN103340855ASlow down disintegrationHigh hardnessAntibacterial agentsPill deliveryAdhesiveSilicon dioxide
The invention relates to a compound amoxicillin and clavulanate potassium tablet and a preparation method thereof. The tablet consists of the following components in percentage by weight: 15 to 30 percent of amoxicillin, 5 to 15 percent of a clavulanate potassium mixture, 30 to 65 percent of a filler, 1.5 to 8 percent of crosslinked povidone, 0.6 to 3 percent of povidone K30, 1 to 3 percent of silica and 1 to 5 percent of glyceryl behenate. The preparation method comprises the following steps: adding the amoxicillin, the filler and the crosslinked povidone into a pelletizer, mixing and adding an adhesive in a mixing process; and adding the prepared amoxicillin granules and clavulanate potassium mixture, the silica and the glyceryl behenate into a mixing machine, mixing, discharging and tabletting. Although the compound amoxicillin and clavulanate potassium tablet adopts a half-wet method to pelletize, the clavulanate potassium is unlikely to degrade, so that the stability of a medicine is guaranteed effectively.
Owner:上海汉维生物医药科技有限公司
Amoxicillin and clavulanate potassium preparation and preparation method thereof
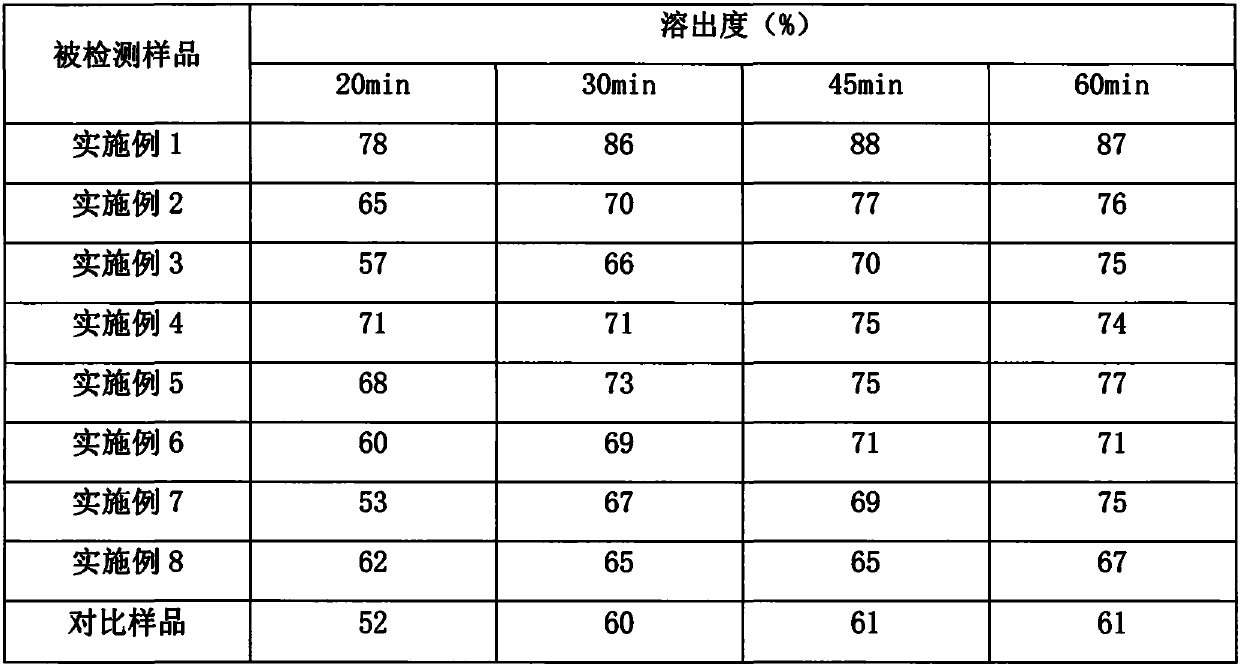
ActiveCN109248150AGood dispersionGood hygroscopicityAntibacterial agentsPharmaceutical non-active ingredientsMedicineCLAVULANATE POTASSIUM
The invention belongs to the technical field medicines, and provides an amoxicillin and clavulanate potassium preparation and a preparation method thereof. According to the dispersed tablet (the preparation) prepared by the technology, the amoxicillin and clavulanate potassium is high in stability, relatively good in dissolution effect and relatively low in related materials. The method provided by the invention can effectively shorten a technological process, reduce a production cycle and explosion time of medicines in time, guarantee stability of medicines and further improve quality of thefinished product (the amoxicillin and clavulanate potassium preparation).
Owner:LUNAN PHARMA GROUP CORPORATION
Beta-cyclodextrin / amoxicillin inclusion compound and its composition with clavulanic kalium and preparation method thereof
InactiveCN100391456CImprove drug stabilityPromote dissolutionAntibacterial agentsMacromolecular non-active ingredientsCellulosePolyethylene glycol
Owner:NANJING J ONE MEDICAL TECH DEV
Method for preparing compound amoxicillin and potassium clavulanate injections
ActiveCN102846606AFast absorptionGood curative effectAntibacterial agentsSolution deliveryAmoxicillin-clavulanate potassiumPhysical chemistry
The invention discloses a novel method for preparing compound amoxicillin and potassium clavulanate injections. The novel method includes the steps of heating oil for injection to 130-150 DEG C, insulating, sterilizing and then cooling the oil for injection to the temperature of 60-90 DEG C; adding aluminium stearate into a part of the sterilized oil for injection, stirring, insulating, cooling to the room temperature after the mixture is uniform, filtering, slowly mixing the mixture with amoxicillin and potassium clavulanate mixture with a certain mass ratio while stirring, and stirring so as to disperse the mixture uniformly until the mixture is completely dispersed into suspension; and refining the mixture via a ball mill, replenishing the sterilized oil for injection to reach a full dose, and continuing stirring till uniform so as to obtain the compound amoxicillin and potassium clavulanate injections. Compared with the prior art, the novel method for preparing the compound amoxicillin and potassium clavulanate injections is simple in preparation process, raw materials and auxiliary materials are low in prices and easy to obtain, the prepared injections are stable in performance and obvious in treatment effect, and is high in cost performance as compared with a like product, the quality of the prepared injections is higher than the quality specified in relative standards of the current pharmacopoeia, and the prepared injections are suitable to be industrially produced in batches.
Owner:SHANGHAI TONGREN PHARM CO LTD
Stable amoxicillin and clavulanate potassium sustained release preparation and preparation technology
ActiveCN102861015AConstant release ratePlay an antibacterial roleAntibacterial agentsPill deliveryAmoxicillin-clavulanate potassiumBiochemistry
The invention provides a stable amoxicillin and clavulanate potassium sustained release preparation, which is composed of a rapid-release layer and a slow-release layer. The weight ratio of amoxicillin to clavulanate potassium is 1000:62.5, the proportion of amoxicillin in the rapid-release layer and the slow-release layer is 1:1, all of clavulanate potassium is placed in the rapid-release layer, wherein the pharmaceutic auxiliary material comprises the following ingredients by weight: the ratio of amoxicillin to slow release material to lubricant to adhesive in the slow release layer is 500:140-150:5-10:16:23, the ratio of amoxicillin to clavulanate potassium to disintegrating agent to lubricant to coating powder in the rapid-release layer is 62.5:30-60:6-10:40-80. The amoxicillin and clavulanate potassium sustained release preparation has the characteristics of good stability, high curative effect, low side-effect incidence rate and reduced drug usage frequency.
Owner:北京乐维生物技术有限公司
Preparation method of amoxicillin and clavulanate potassium tablets
InactiveCN103961351AEasy to prepareAddress controllabilityPharmaceutical delivery mechanismPharmaceutical non-active ingredientsChemical synthesisAdditive ingredient
The invention belongs to the field of chemosynthesis, and particularly relates to a preparation method of amoxicillin and clavulanate potassium tablets. The preparation method comprises the following steps: mixing major ingredients, mixing minor ingredients, pressing particles, sieving, repeatedly pressing particles, tabletting, coating and the like. The preparation method enhances the control to the pelletizing and tabletting processes of the amoxicillin and clavulanate potassium tablets, introduces a novel coating technology to overcome the defects of poor humidity controllability of the amoxicillin and clavulanate potassium tablets and product instability, ensures the quality of drugs, and improves the production efficiency; the steps of crushing, sieving and selecting fine powders to be pressed into particles are repeated.
Owner:KANGTIAN PHARMA ZHONGSHAN
Injection-use pharmaceutical composition comprising cefmetazole sodium and clavulanate potassium
ActiveCN103271925AEnhanced inhibitory effectGood resolubilityAntibacterial agentsOrganic active ingredientsGlycineMANNITOL/SORBITOL
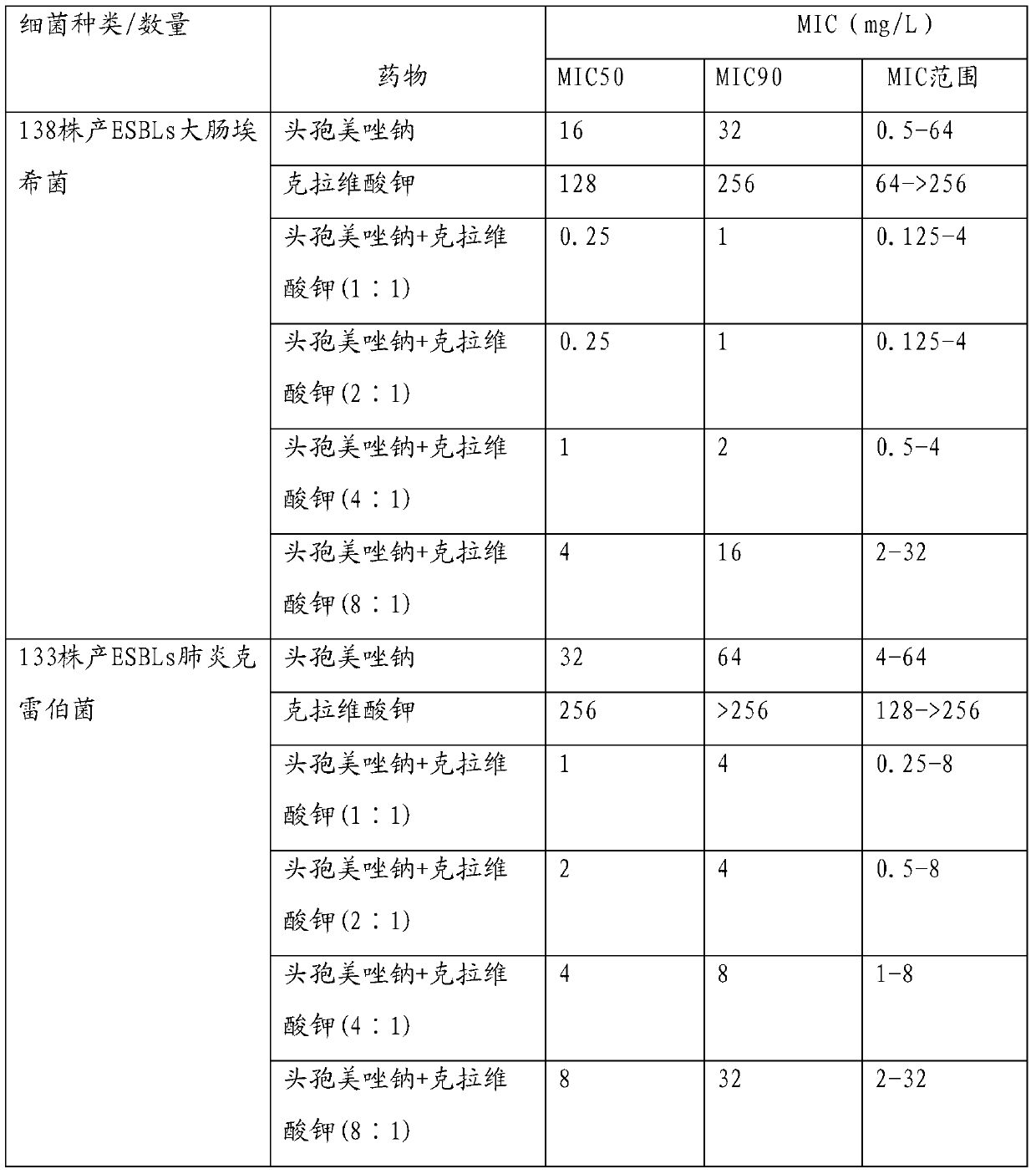
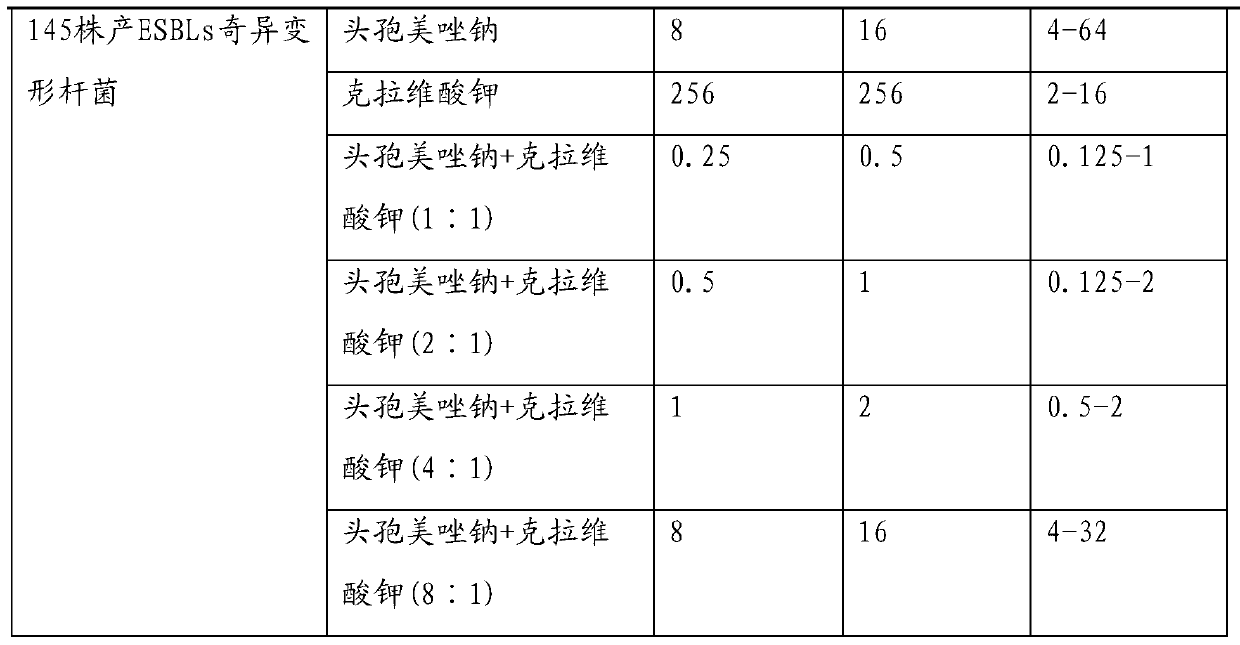
The invention provides an injection-use pharmaceutical composition comprising cefmetazole sodium and clavulanate potassium, and the pharmaceutical composition has a cooperatively antibacterial effect. The pharmaceutical composition comprises the following ingredients: in parts by weight, 1-4 parts of the cefmetazole sodium; 1-2 parts of the clavulanate potassium; 2-6 parts of polyalkylcyanoacrylate; 0.2-0.5 parts of L-arginine; 0.5-1 parts of PVP; 0.5-1 parts of mannitol; 0.5-1 parts of glycine; 1-2 parts of a tween. The pharmaceutical composition has the advantages of high stability, good redissolubility, simple preparation processes, a good inhibitory effect on ESBLs-producing escherichiacoli, klebsiellapeumoniae, and proteusmirabilis, and a definite curative effect, can reduce the dosage of a single prescription preparation and shorten the treatment cycle, and has broad market prospects.
Owner:FUAN PHARM (GRP) CO LTD +1
Amoxicillin and clavulanate potassium preparations and preparation method thereof
InactiveCN110051637AEasy to produceImprove bioavailabilityAntibacterial agentsPharmaceutical non-active ingredientsSpray GranulationWater soluble
The invention relates to amoxicillin and clavulanate potassium preparations and preparation method thereof. Amoxicillin and clavulanate potassium can be prepared into two preparations including that amoxicillin and clavulanate potassium are mixed and dissolved with a certain amount of water-soluble matrixes and then prepared into dropping pills through dropping pill preparation technology; or, amoxicillin and additives are micro-ground and uniformly mixed, then clavulanate potassium is dissolved in a certain amount of water into binding agent, and through spraying granulation, the materials are prepared into granules. The amoxicillin and clavulanate potassium preparations prepared through the methods above have the advantages of being simple in production, high in bioavailability, rapid inabsorption and effects and the like.
Owner:葵花药业集团北京药物研究院有限公司
Preparation method of amoxicillin sodium and clavulanate potassium for injection
InactiveCN104173340ASimple production processImprove product qualityAntibacterial agentsPowder deliveryAmoxicillin SodiumPowder injection
The invention provides a preparation method of amoxicillin sodium and clavulanate potassium for injection. An amoxicillin sodium and clavulanate potassium powder-injection manufactured by using the method disclosed by the invention is simple in production process, safe and reliable, and stable in product quality, and the period of validity is up to 24 months, therefore, the amoxicillin sodium and clavulanate potassium powder-injection is applicable to mass industrial production.
Owner:SICHUAN PHARMA
Electrolyte for secondary battery and preparation method thereof
ActiveCN107666012AImprove performanceImprove stabilityFinal product manufactureLead-acid accumulators constructionPorphyrinSodium bisulfate
The invention discloses electrolyte for a secondary battery. The electrolyte is prepared from the following raw materials including acid sulfate, solvents and additives, wherein the acid sulfate is prepared by mixing sodium hydrogensulfate and potassium hydrogensulfate; the solvents are one kind of materials or a composition of a plurality of kinds of materials from water, maleic acid, tetrahydrofurfuryl alcohol, a boric acid solution, porphyrin, acrylic acid, dibutyl carbonate, isoamyl acetate, povidon iodine, triethyl phosphate and water glass; the additives are prepared from the following raw materials including lithium borate oxalate, glycol sulfite, sodium chromate, carboxymethyl chitosan, sodium selenite and potassium clavulanate. The electrolyte for the secondary battery has the advantages that each performance completely conforms to the national standard; the defects of long formation time, non-thorough formation and poor uniformity in the ordinary lead acid storage battery canbe successfully overcome; the stability of the battery is greatly improved.
Owner:江苏海涛新能源科技有限公司
Method for measuring amoxicillin content of amoxicilin sodium and clavulanate potassium
InactiveCN104266988AEliminate distractionsAccurate measurementColor/spectral properties measurementsAmoxycillin sodiumAnalytical chemistry
The invention provides a method for measuring amoxicillin content of amoxicilin sodium and clavulanate potassium. The interference of clavulanate potassium can be eliminated in an environment with certain pH values and in a certain wavelength range, and the measurement result can be accurate; and the method is simple and convenient and can provide support for controlling the acceptable quality of the amoxicilin sodium and clavulanate potassium in production.
Owner:SICHUAN PHARMA
Amoxicillin and clavulanate potassium dispersible tablet
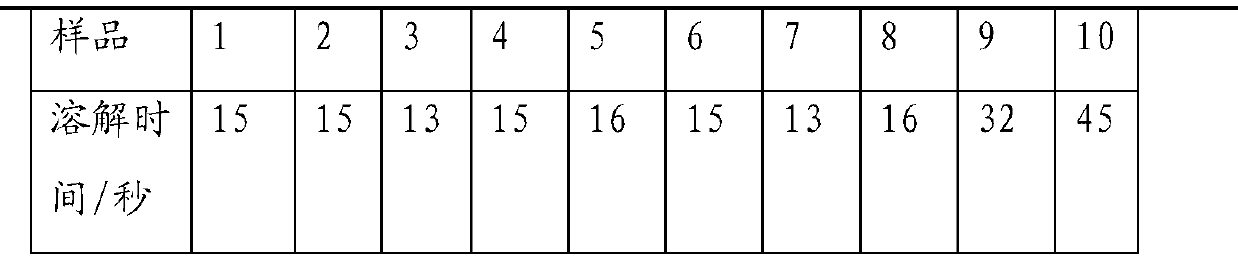
ActiveCN103768056ADisintegrates quicklyImprove stabilityAntibacterial agentsPharmaceutical non-active ingredientsNuclear chemistryDispersible tablet
The invention discloses an amoxicillin and clavulanate potassium dispersible tablet applied to the field of a preparation of the amoxicillin and clavulanate potassium dispersible tablet. The dispersible tablet is prepared from the following components: amoxicillin trihydrate, clavulanate potassium, microcrystalline cellulose, a disintegrant, diatomite, a sweetener and an aromatic, wherein the disintegrant is selected from one of croscarmellose sodium, carboxymethyl starch sodium and polyvinylpolypyrrolidone; the disintegrant is preferably selected from croscarmellose sodium; the sweetener is selected from one of aspartame and sucralose; the aromatic is selected from one of a blueberry flavor and a lemon essence; the sweetener is preferably selected from sucralose; the aromatic is preferably selected from the blueberry flavor; the weight ratio of amoxicillin trihydrate to clavulanate potassium in the dispersible tablet is 4:1 based on amoxicillin and clavulanate; the dispersible tablet is prepared from powder by a direct compression method. The amoxicillin and clavulanate potassium dispersible tablet is rapid to disintegrate, still can be rapidly disintegrated at low water temperature, and is good in stability, good in mouthfeel, simple in prescription, good in mobility of mixed powder in the prescription, and not sticking in the tabletting process.
Owner:NORTHEAST PHARMA GRP SHENYANG SHIDE PHARMA
Novel method for preparing clavulanate
ActiveCN101279982ASignificantly progressiveSolving the Recycling ConundrumAntibacterial agentsOrganic chemistryDicarbonateBicarbonate preparation
The invention relates to biological medicine field and in particular relates to a new method to prepare pharmaceutically acceptable salt from fermentation broth of streptomyces sp. The method directly uses alkali carbonate and bicarbonate to prepare clavulanate potassium or other pharmaceutically acceptable salt without producing intermediate compound clavulanic acid. The invention is simple in process and is suitable for industrial production.
Owner:LUNAN PHARMA GROUP CORPORATION
Liposome injection of pharmaceutical composition of amoxicillin sodium and clavulanate potassium
InactiveCN101804051BImprove stabilityHigh encapsulation efficiencyAntibacterial agentsPharmaceutical non-active ingredientsAntioxidantFreeze-drying
The invention provides a liposome injection of a pharmaceutical composition of amoxicillin sodium and clavulanate potassium, which consists of amoxicillin sodium, clavulanate potassium, a liposome carrier, a lyoprotectant and a freely optional antioxidant, wherein the liposome carrier is egg yolk phosphatidylinositol and sodium taurocholate. The liposome injection has good preparation stability, liposome can avoid the fracture due to dehydration, fusion, ice crystal generation and the like during the freeze-drying process, and the liposome can still keep the good encapsulation rate after hydration and redissolution.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Medicinal composition, as well as preparation process and application thereof
ActiveCN102846605AReduce decompositionRestore sensitivityAntibacterial agentsRespiratory disorderDiseasePharmaceutical Substances
The invention discloses a medicinal composition, which comprises the following components in parts by weight: 5 to 15 parts of amoxicillin, 1 to 5 parts of clavulanate potassium, and 1 to 5 parts of ambroxol hydrochloride. The invention discloses an application of the medicinal composition in preparing medicaments for treating animal respiratory infectious diseases, in particular for preparing porcine infectious pleuropneumonia and haemophilusparasuis. The invention also discloses a preparation method of compound amoxicillin soluble powder. The compound amoxicillin soluble powder comprises the following components in parts by weight: 5 to 15 parts of amoxicillin, 1 to 5 parts of clavulanate potassium, 1 to 5 parts of ambroxol hydrochloride, and 75 to 93 parts of glucosum anhydricum. The raw materials are uniformly mixed according to an equivalent incremental principle to obtain the compound amoxicillin soluble powder. The medicinal composition disclosed by the invention is reasonable and scientific in prescription, the bioavailability of amoxicillin can be increased compared with an amoxicillin preparation, the drug resistance is reduced, and the curative effect is improved.
Owner:JIUJIANG DACHENG PHARMA CO LTD
Compositions and methods of treatment comprising amoxicillin and potassium clavulanate with xanthan
Owner:GLAXO GRP LTD
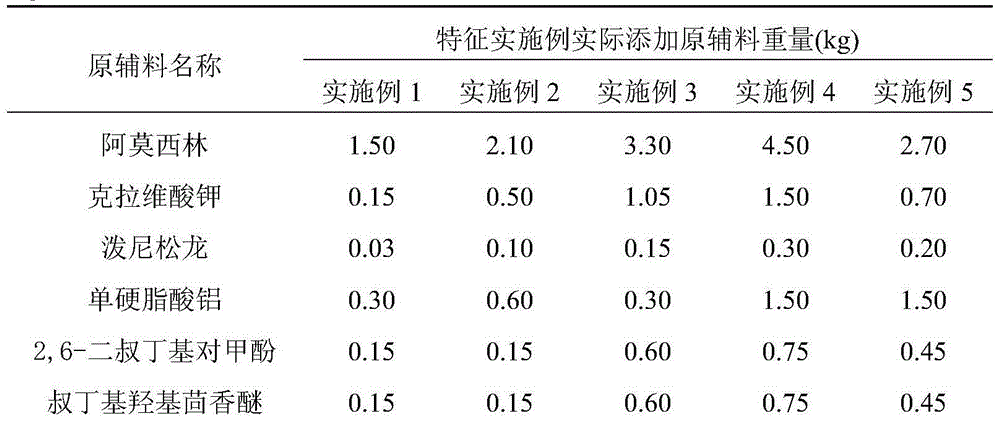
Compound amoxicillin breast injectant prescription and preparation method thereof
ActiveCN104415040AGuaranteed uniformityGuaranteed stabilityAntibacterial agentsAntipyreticPenicillinAntioxidant
The invention relates to a compound amoxicillin breast injectant prescription and a preparation method thereof. The compound amoxicillin breast injectant prescription comprises a suspending aid, an antioxidant and a solid dispersion carrier which are prepared through a special process to obtain white to off-white oily suspension. The compound amoxicillin breast injectant prescription disclosed by the invention has the advantages of simple preparation process, stable content, easy injection and the like. The composition disclosed by the invention is compounded from amoxicillin, clavulanate potassium and prednisolone; compared with other amoxicillin patent preparations, the composition has drug-tolerant bacteria for penicillins, and also has a high inhibition effect on the chromosome exzymes generated by klebsiella peneumoniae, bacillus proteus vulgaris and bacteroides fragilis; prednisolone has anti-inflammatory and antiallergic functions, and has a synergistic effect on bacterial infection and allergic inflammation.
Owner:RINGPU TIANJIN BIOLOGICAL PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com