Pharmaceutical composition containing amoxicillin trihydrate and clavulanate potassium
A technology of amoxicillin trihydrate and potassium clavulanate, which is applied in the field of novel sustained-release pharmaceutical preparations and can solve the problems of no pharmaceutical-grade product supply and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
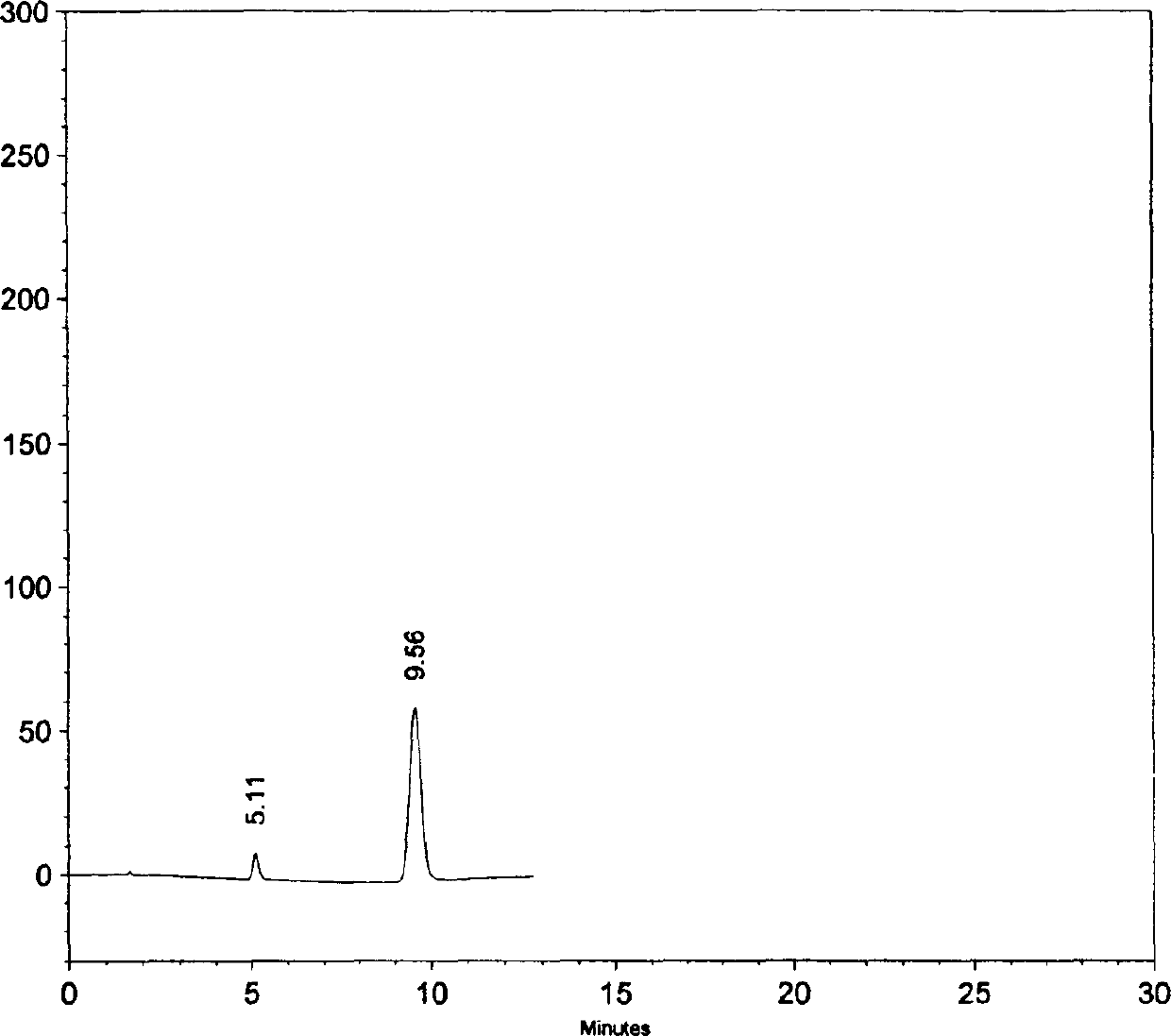
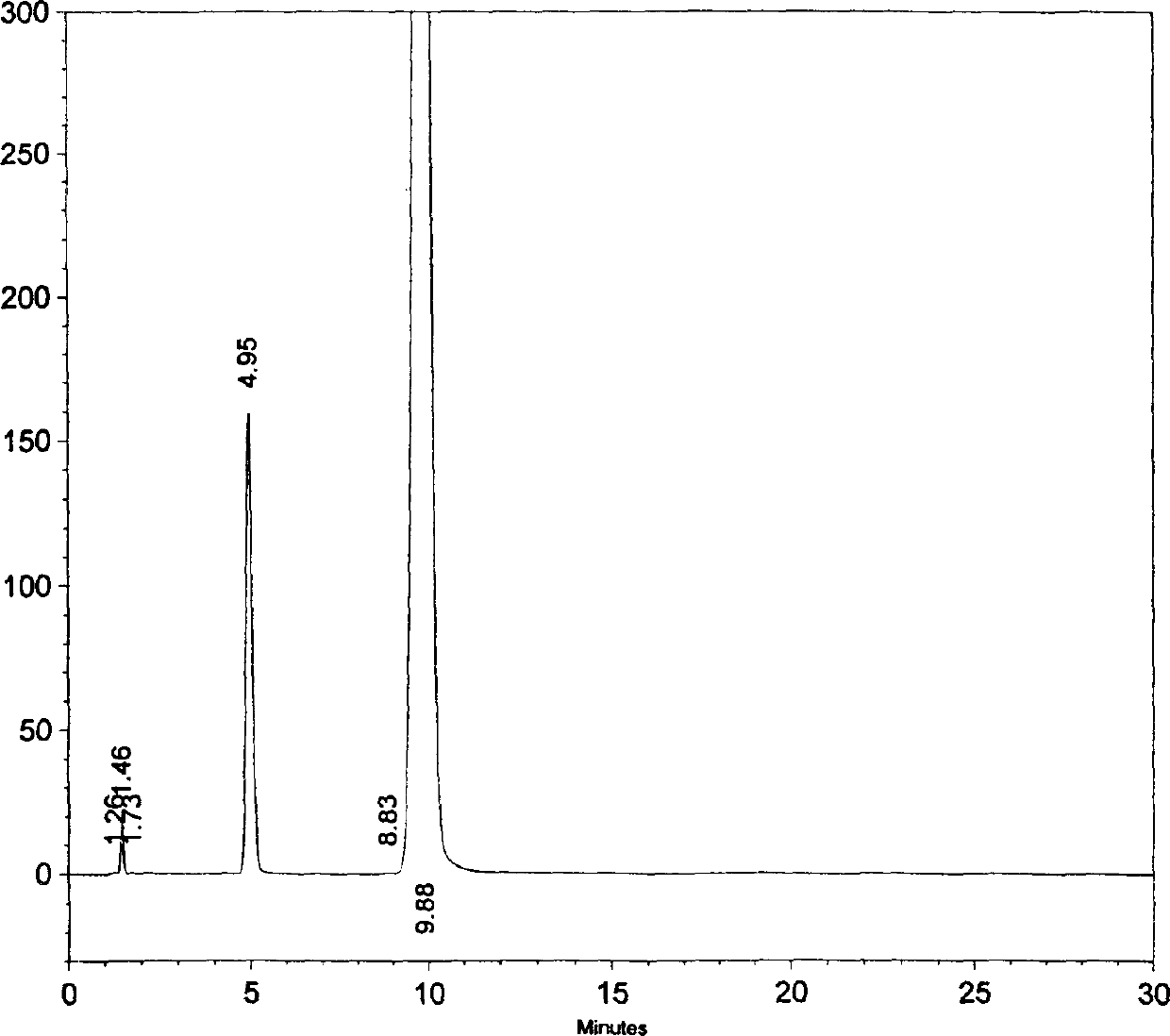
Image
Examples
Embodiment 1
[0046] The modified release formulation containing amoxicillin trihydrate and clavulanic acid potassium (weight ratio is 16: 1) of the present invention is a sustained-release three-layer tablet, wherein the weight is based on amoxicillin anhydrous and clavulanic acid respectively, Layer 1 is an immediate release layer containing potassium clavulanate and part of amoxicillin trihydrate; layer 2 is an immediate release layer containing part of amoxicillin trihydrate; layer 3 is a sustained release layer containing the rest of amoxicillin trihydrate.
[0047] The prescription is as follows:
[0048] Potassium Amoxicillin Clavulanate (2:1)
[0049] (Based on amoxicillin anhydrous and clavulanic acid) 93.75g
[0050] Layer 1 sodium starch glycolate 7.0g
[0051] Magnesium Stearate 2.0g
[0052] Micronized silica gel 1.0g
[0053]Amoxicillin trihydrate (calculated as anhydrous substance) 161.5g
[0054] Microcrystalline Cellulose 40.0g
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com