Preparation method of amoxicillin and clavulanate potassium tablets
A technology of amoxicillin-clavulanate potassium and clavulanate potassium, which is applied in the direction of non-active ingredient medical preparations, active ingredient-containing medical preparations, pharmaceutical formulas, etc., and can solve poor humidity controllability and product instability and other issues to achieve the effect of ensuring quality, reducing dosage, and reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] A kind of preparation method of amoxicillin-clavulanate potassium tablet, obtain through following steps:
[0024] Prescription of amoxicillin and clavulanate potassium tablets: 875g of amoxicillin, 125g of potassium clavulanate, 226.6g of microcrystalline cellulose, 10.0g of micronized silica gel, 29.0g of croscarmellose sodium, and 14.5g of magnesium stearate. Among them, the internal part of microcrystalline cellulose is 212.1g, and the external part is 14.5g; the internal part of magnesium stearate is 7.25g, and the external part is 7.25g.
[0025] The above raw materials can be used for the production of 1000 amoxicillin and clavulanate potassium tablets. The coating material used to produce amoxicillin-clavulanate potassium tablets is Opadry white OY-C-7000A of Colorcon Company. The coating powder consists of hypromellose 2910 (5CPS), titanium dioxide, ethyl cellulose (10CPS), and diethyl phthalate.
[0026] Concrete preparation process is as follows:
[0027] ...
Embodiment 2
[0036] A kind of preparation method of amoxicillin-clavulanate potassium tablet, obtain through following steps:
[0037] Prescription of amoxicillin and clavulanate potassium tablets: 875g of amoxicillin, 125g of potassium clavulanate, 337.5g of microcrystalline cellulose, 12.5g of micronized silica gel, 12.5g of croscarmellose sodium, and 25g of magnesium stearate. Among them, the internal part of microcrystalline cellulose is 315.9g, and the external part is 21.6g; the internal part of magnesium stearate is 12.5g, and the external part is 12.5g.
[0038] The above raw materials can be used for the production of 1000 amoxicillin and clavulanate potassium tablets. The coating material used to produce amoxicillin-clavulanate potassium tablets is Opadry white OY-C-7000A of Colorcon Company. The coating powder consists of hypromellose 2910 (5CPS), titanium dioxide, ethyl cellulose (10CPS), and diethyl phthalate.
[0039] The specific preparation process is the same as in Examp...
Embodiment 3
[0042] Prescription of amoxicillin and clavulanate potassium tablets: 875g of amoxicillin, 125g of potassium clavulanate, 266.7g of microcrystalline cellulose, 25g of micronized silica gel, 25g of croscarmellose sodium, and 33.3g of magnesium stearate. Among them, the internal part of microcrystalline cellulose is 249.6g, and the external part is 17.1g; the internal part of magnesium stearate is 16.7g, and the external part is 16.6g.
[0043] The above raw materials can be used for the production of 1000 amoxicillin and clavulanate potassium tablets. The coating material used to produce amoxicillin-clavulanate potassium tablets is Opadry white OY-C-7000A of Colorcon Company. The coating powder consists of hypromellose 2910 (5CPS), titanium dioxide, ethyl cellulose (10CPS), and diethyl phthalate.
[0044] The specific preparation process is the same as in Example 1.
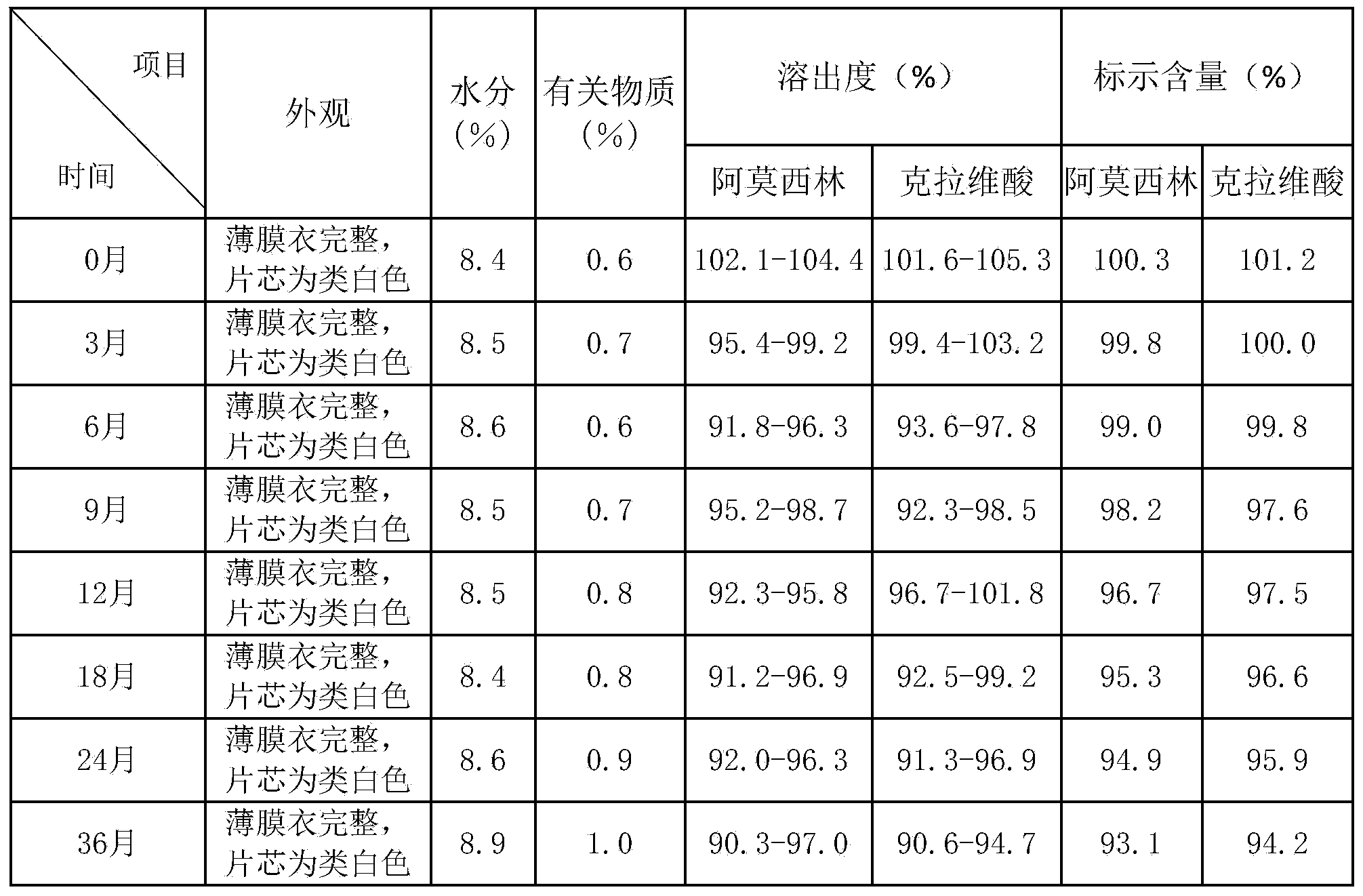
[0045] The amoxicillin / potassium clavulanate 7:1 sheet prepared by the above method meets the requirements of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com