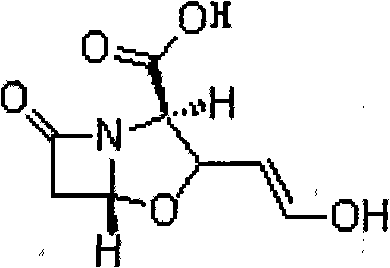
Novel method for preparing clavulanate
A technology of clavulanate and clavulanic acid, which is applied in the field of biomedicine, can solve the problems of increasing the difficulty of solvent recovery, and achieve the effects of avoiding solvent recovery problems, reducing dosage, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Ethyl acetate extract of clavulanic acid, dehydrated and decolorized. K 2 CO 3 (1.0 equivalent) dissolved in pure water to prepare a 60% solution (m / v), slowly added to the above extract, the mixture was stirred at 5°C for 45 minutes, and allowed to stand for layers. The aqueous phase containing clavulanic acid was collected, 30 times the volume of isopropanol was slowly added, and the mixture was stirred at 5° C. for 120 minutes. The crystalline product was filtered and the crystals were washed with acetone. The crystals were dried in a vacuum dryer (30° C.) for about 2 hours to obtain 240 g of potassium clavulanate (yield 78%).
Embodiment 2
[0021] Methyl acetate extract of clavulanic acid, dehydrated and decolorized. KHCO3 (1.1 equivalent) was dissolved in pure water to make a 30% solution (m / v), and the above extract was slowly added, the mixture was stirred at 5° C. for 45 minutes, and allowed to stand to separate into layers. The aqueous phase was collected, and the aqueous solution containing clavulanic acid was slowly added with 30 times the volume of isopropanol, and the mixture was stirred at 5°C for 120 minutes. The crystalline product was filtered and the crystals were washed with acetone. The crystals were dried in a vacuum dryer (30° C.) for about 2 hours to obtain 218 g of potassium clavulanate (yield 71%).
Embodiment 3
[0023] Methyl ethyl ketone extract of clavulanic acid, dehydrated and decolorized. K 2 CO3 (1.2 equivalents) was dissolved in pure water to make a 50% solution (m / v), and the above extract was added slowly, the mixture was stirred at 5°C for 45 minutes, and the layers were separated. The aqueous phase was collected, and the aqueous solution containing clavulanic acid was slowly added with 35 times the volume of isopropanol, and the mixture was stirred at 5° C. for 120 minutes. The crystalline product was filtered and the crystals were washed with acetone. The crystals were dried in a vacuum dryer (30° C.) for about 2 hours to obtain 250 g of potassium clavulanate (yield 82%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com