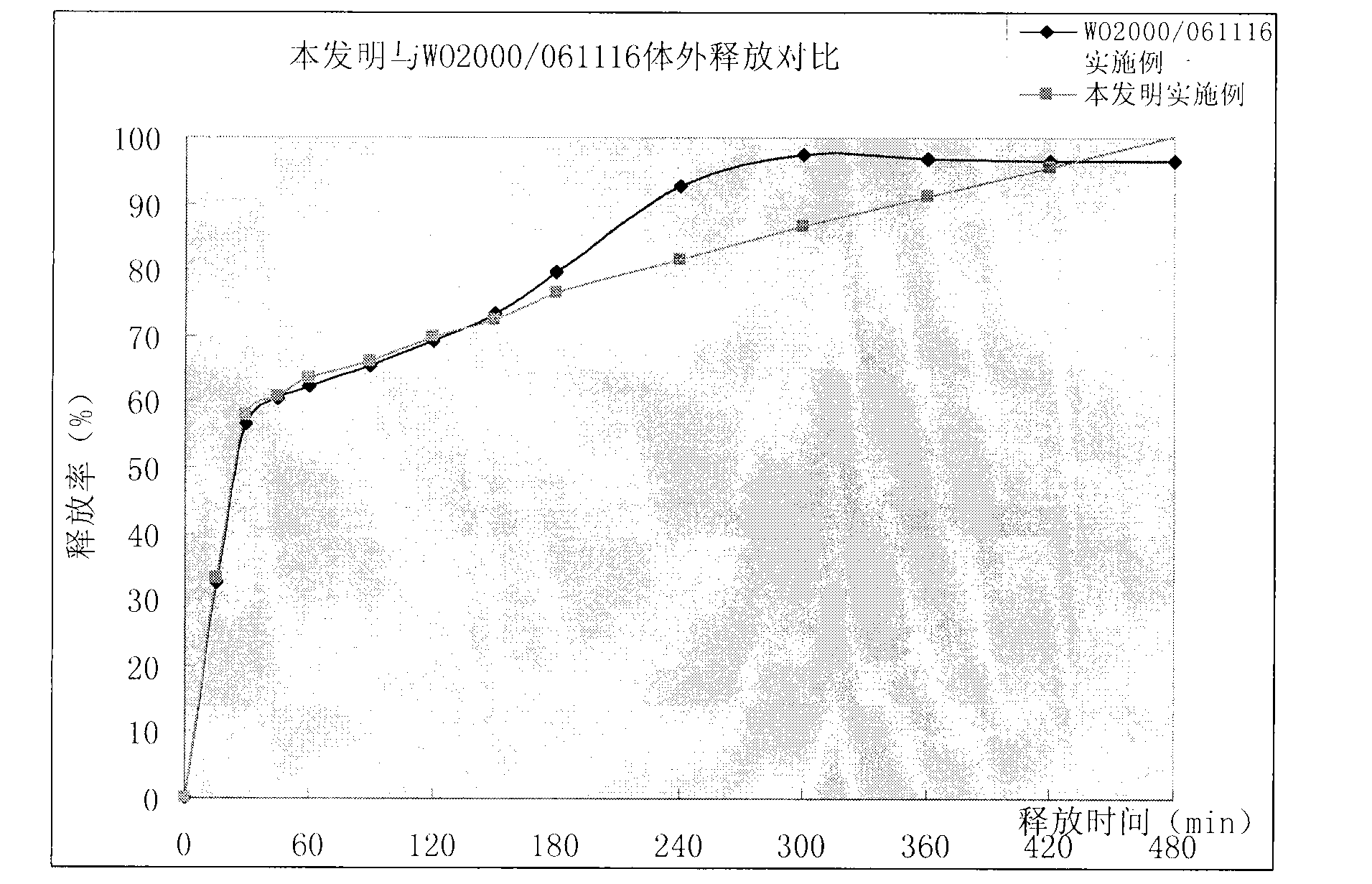
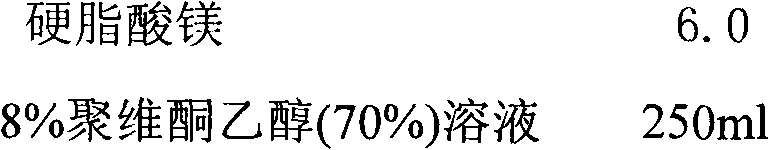Stable amoxicillin and clavulanate potassium sustained release preparation and preparation technology
A technology of potassium clavulanate and slow-release preparations, which is applied to medical preparations containing active ingredients, pill delivery, antibacterial drugs, etc. It can solve the problems of increased incidence and loss of efficacy of antibiotics, and reduce the number of medications. Constant release rate and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Slow release layer:
[0027]
[0028]
[0029] Immediate release layer:
[0030]
[0031] The preparation method of the amoxicillin delayed-release preparation of the present invention is as follows, comprising: the quick-release layer amoxicillin and all potassium clavulanate and auxiliary materials are granulated by a dry method, and the slow-release layer amoxicillin and the sustained-release materials are prepared by a wet method. Granules, and finally use a double-layer tablet press for tableting and coating.
[0032] The preparation method of the preparation is as follows: the slow-release layer is granulated by wet method, the main drug amoxicillin and the slow-release material are mixed evenly, and then the adhesive is added, wet granulated, dried, and granulated to obtain the sustained-release layer granules for later use; Release layer: Mix the main drug amoxicillin and potassium clavulanate with auxiliary materials evenly, dry granulate, and dry und...
Embodiment 2
[0036] Slow release layer:
[0037]
[0038] Immediate release layer:
[0039]
[0040]
[0041] The preparation method is the same as in Example 1.
Embodiment 3
[0043] Slow release layer:
[0044]
[0045] Immediate release layer:
[0046]
[0047] The preparation method is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com