Method for preparing indapamide sustained-release preparation
A technology of indapamide and sustained-release agent, which is applied in the field of pharmaceutical processing, can solve the problems of drug recrystallization, unfavorable safety production and environmental protection, and high risk factor, so as to avoid the loss of active ingredients and benefit safe production and environment Protect and avoid cross-contamination effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] A preparation method of indapamide sustained-release agent, comprising the steps of:
[0025] (1) Place the microcrystalline cellulose, lactose, indapamide and starch in the formula dosage in a high-speed mixing granulator for premixing for 3-10 minutes to obtain a premix.
[0026] (2) The acrylic resin, triethyl citrate and talcum powder of formula consumption are dispersed in appropriate amount of water, and stirred evenly to form a suspension with a solid content of 5 to 15wt.%. Acrylic resin is selected for use in the following specific examples here Eudragit NE30D, its solid content is 30wt.%.
[0027] (3) Spray 50-90wt.% of the suspension in (2) into (1) in the form of atomization under the condition that the stirring speed of the high-speed mixing granulator is 300-800rpm and the cut-off speed is 1200-3000rpm In the premix, then reduce the stirring speed to 150-300rpm, add the remaining suspension, and continue to stir to prepare sustained-release pellets with a...
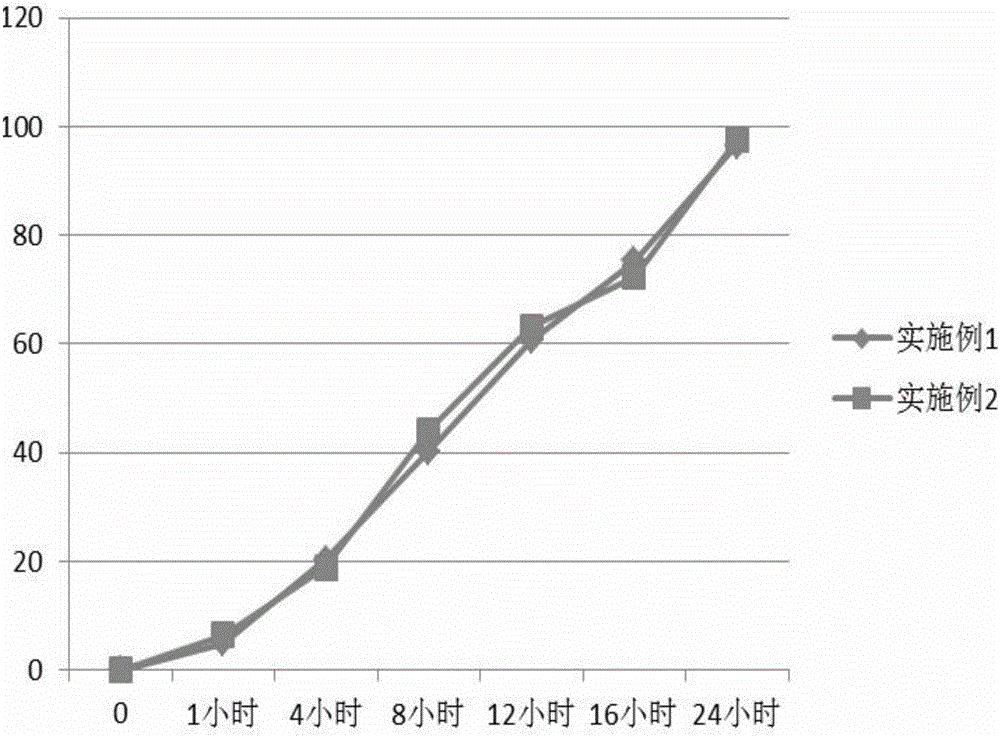
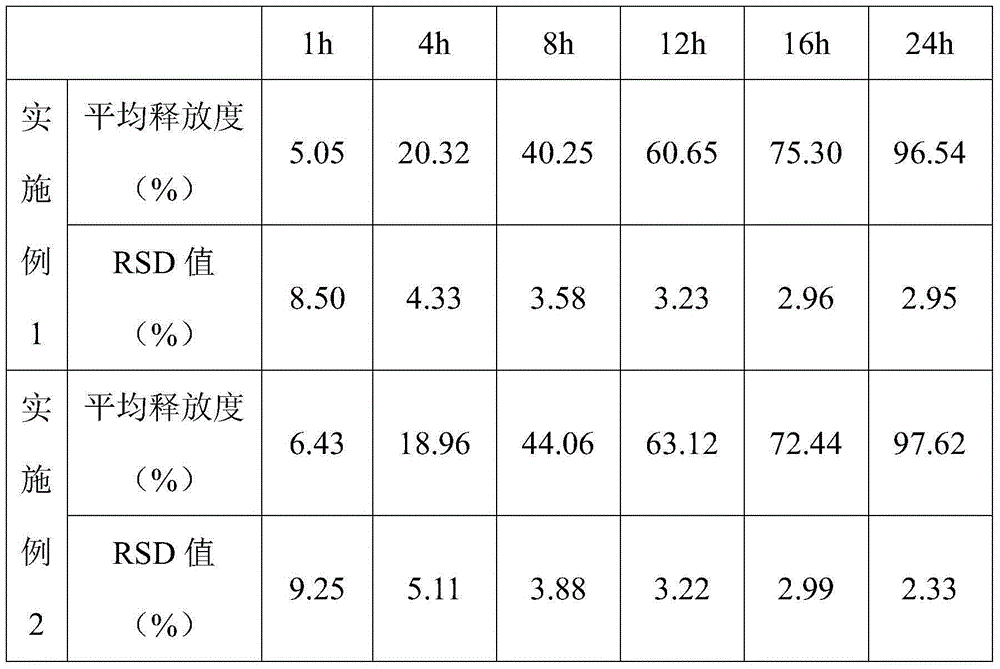
specific Embodiment 1
[0033] (1) Put 250g of microcrystalline cellulose, 250g of lactose, 15g of indapamide and 100g of starch in a high-speed mixing granulator, turn on the stirring paddle and premix for 5 minutes to obtain a premix.
[0034] (2) Disperse 1000g of acrylic resin Eudragit NE30D (30wt.% solid content), 10g triethyl citrate and 50g talcum powder in an appropriate amount of water, and stir evenly to form a suspension with a solid content of 10wt.%.
[0035] (3) 70wt.% of the suspension in (2) is sprayed into the premix in (1) in atomized mode under the state that the stirring speed of the high-speed mixing granulator is 500rpm and the cutting speed is 2000rpm, and then Reduce the stirring speed to 250rpm, add the remaining suspension, and continue to stir at a speed of 150rpm to prepare slow-release pellets with a particle size of 100-1000μm, which are then transported to a fluidized bed through a material pipeline and dried until the water content is less than 2%, the slow-release pel...
specific Embodiment 2
[0037] (1) Put 250g of microcrystalline cellulose, 250g of lactose, 15g of indapamide and 100g of starch in a high-speed mixing granulator, turn on the stirring paddle and premix for 10 minutes to obtain a premix.
[0038](2) Disperse 1000g of acrylic resin Eudragit NE30D (30wt.% solid content), 10g triethyl citrate and 50g talcum powder in an appropriate amount of water, and stir evenly to form a suspension with a solid content of 15wt.%.
[0039] (3) 70wt.% of the suspension in (2) is sprayed into the premix in (1) in atomized mode under the state that the stirring speed of the high-speed mixing granulator is 500rpm and the cutting speed is 2000rpm, and then Reduce the stirring speed to 250rpm, add the remaining suspension, and continue to stir at a speed of 150rpm to prepare slow-release pellets with a particle size of 100-1000μm, which are then transported to a fluidized bed through a material pipeline and dried until the water content is less than 2%, the slow-release pel...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com