Preparation method of amoxicillin sodium and clavulanate potassium for injection
A technology for amoxicillin sodium clavulanate potassium and clavulanate potassium, which is applied in the pharmaceutical field, can solve problems such as difficulty in unifying quality standards, difficulty in removing organic solvents, changes in physical properties of fluids, etc., and achieves stable product quality and simple production process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0047] The present invention will be further illustrated below by way of examples, but the present invention is not limited thereto. For the experimental methods without specific conditions indicated in the following examples, the conventional conditions or the conditions suggested by the manufacturer are usually followed. Unless otherwise specified, the percentages presented below are all mass percentages.
[0048] See Table 1 for the list of materials used (1.2g specification).
[0049] Table 1
[0050]
[0051] See Table 2 for the operating room and main equipment of each process.
[0052] Table 2
[0053]
[0054] 1. Raw material preparation:
[0055] (1) Check the name, batch number and quantity of raw materials.
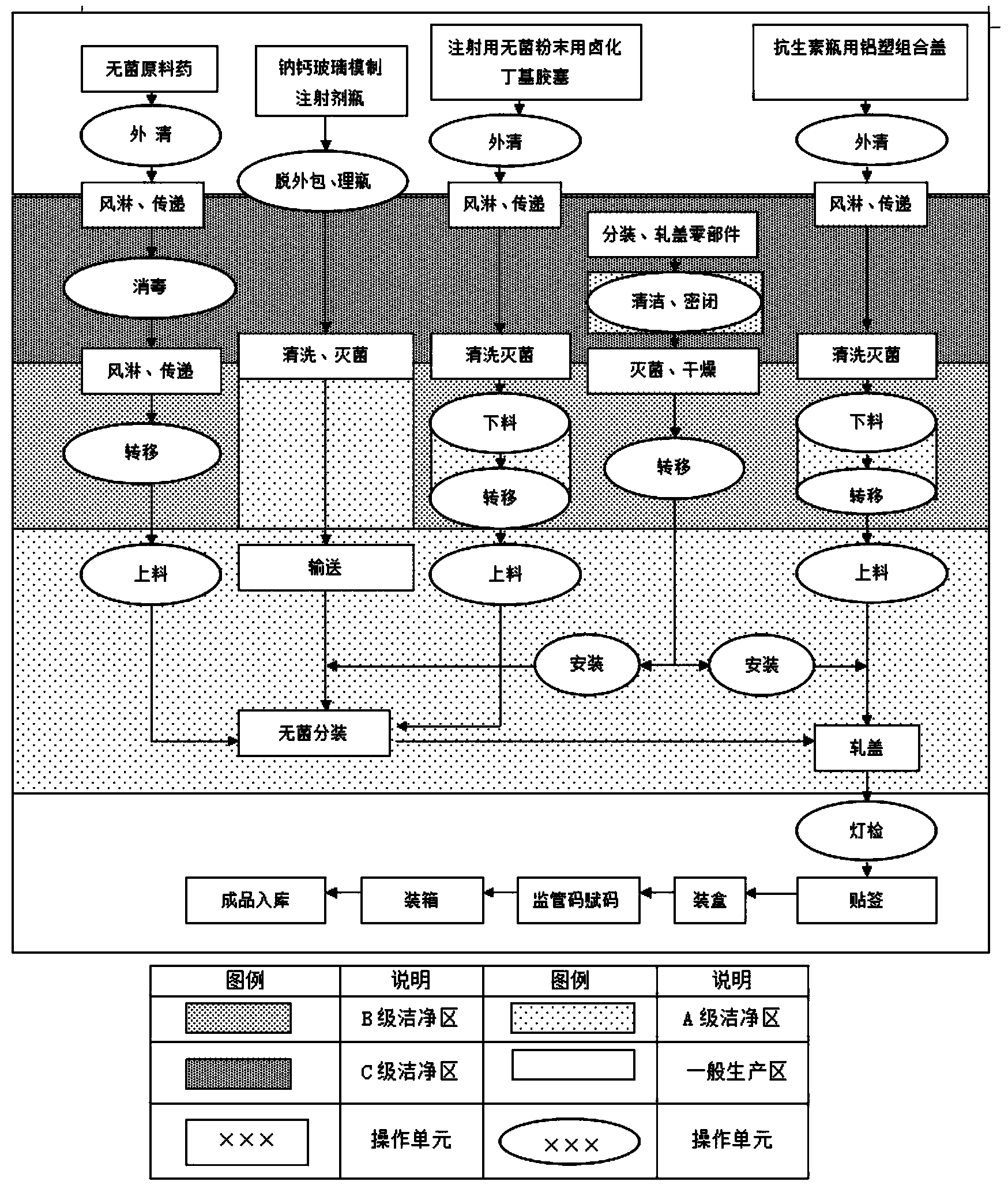
[0056] (2) According to figure 2 The process flow shown is for raw material delivery.
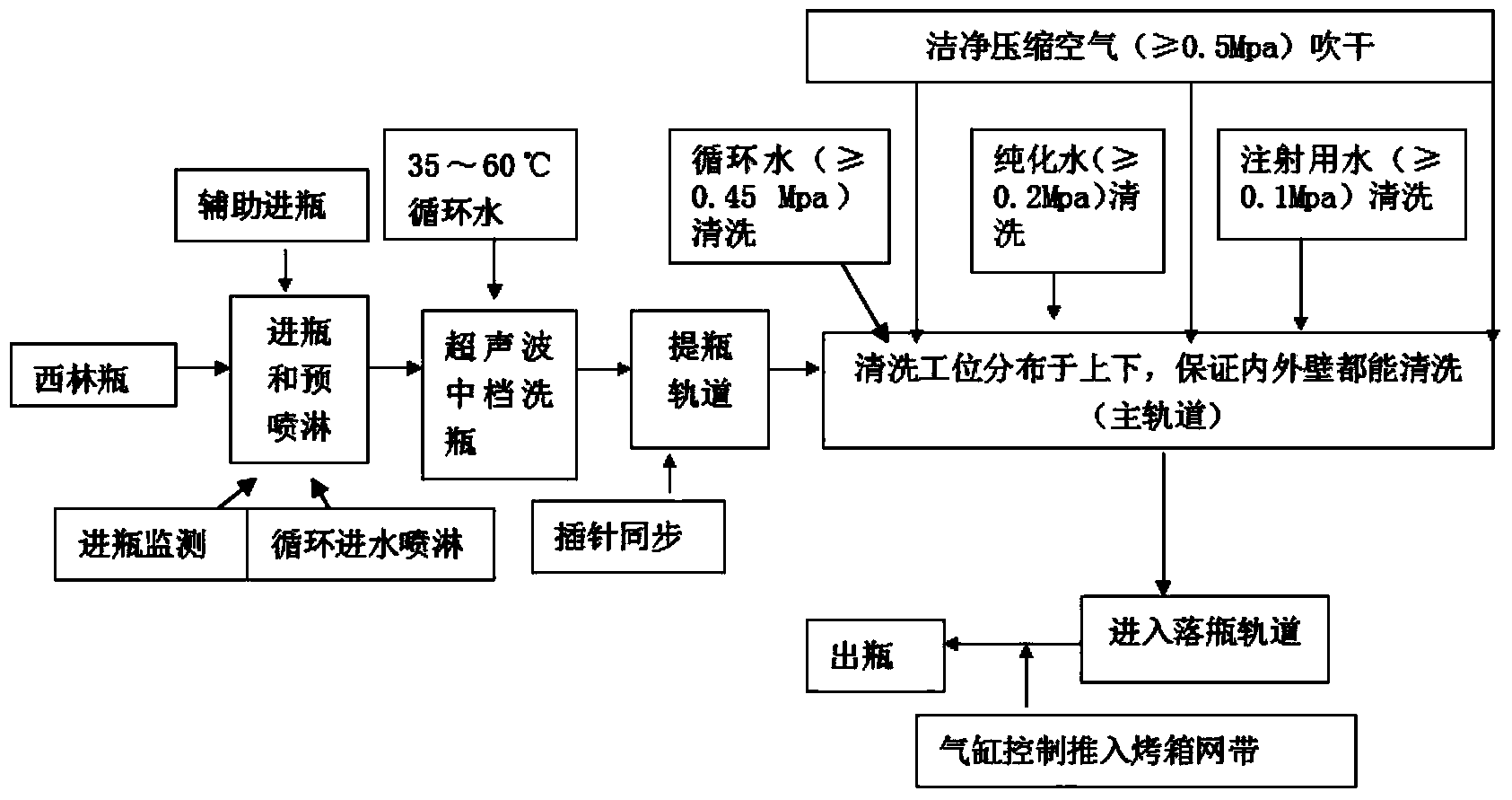
[0057] 2. Cleaning and sterilization of soda-lime glass molded injection bottles:
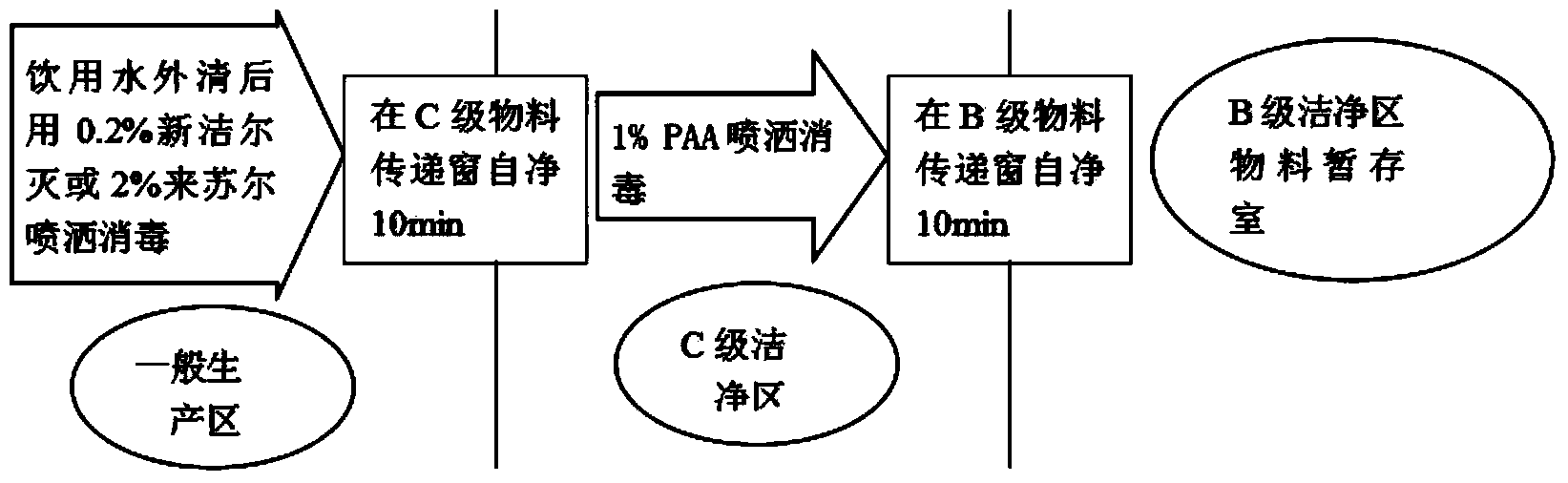
[0058] (1) Soda-lime glass molded injection bottle is unpacked to check th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com