Method for preparing compound amoxicillin and potassium clavulanate injections
A technology of amoxicillin-clavulanate potassium and clavulanate potassium, which is applied in the preparation of compound amoxicillin-clavulanate potassium preparations and compound amoxicillin-clavulanate potassium injections, which can solve the problems of high price and limited Issues such as the scope of application, to achieve the effect of easy absorption and utilization, improved stability and drug effectiveness, and less irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
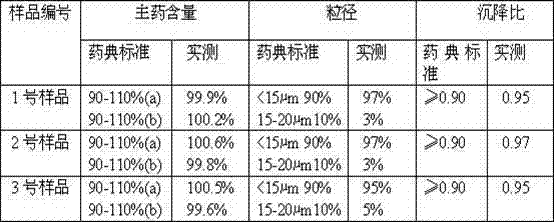
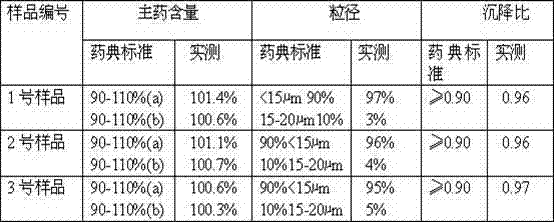
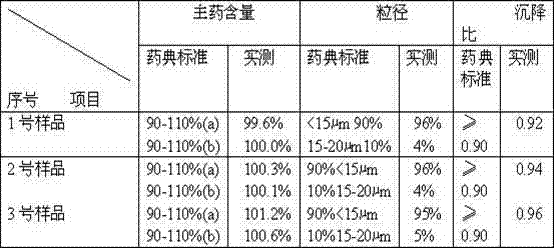
[0025] Weigh 14.0g of amoxicillin and 3.5g of potassium clavulanate, put them into a pulverizer and pulverize until more than 90% of the particle size is ≤15-20μm; take 80mL of injection-grade soybean oil, heat it to 130°C in a container, stir and sterilize it for 50 minutes, then cool to 60°C, add 1g of injection-grade aluminum stearate to 20mL of soybean oil after cooling, heat and stir to disperse evenly, cool to 20°C, filter the oil gel through an 80-mesh metal filter; under stirring (speed: 100r / min) slowly add the crushed amoxicillin and potassium clavulanate, stir and disperse evenly, and completely disperse into a suspension; ultrafine the obtained suspension through a homogenizer, and put the suspension into In the four stainless steel containers in the high-performance ball mill, start the ball mill for 5-10 minutes, and make the suspension fine to make it ultra-fine (95% or more to 15μm or less), and make up for the injection cooled to 20 ℃ Soybean oil to a suffici...
Embodiment 2
[0032] Weigh 14.0g of amoxicillin and 3.5g of potassium clavulanate, put them into a pulverizer and pulverize until more than 90% of the particles have a particle size of ≤15-20μm; take 83mL of injection-grade soybean oil, heat it to 140°C in a container, stir and sterilize it for 65 minutes, then cool To 65°C, add 1.5g of injection-grade aluminum stearate to 30mL of soybean oil after cooling, heat and stir to disperse evenly, cool to 28°C, filter the oil glue through a 120-mesh metal filter; under stirring (speed: 125r / min) slowly add the crushed amoxicillin and potassium clavulanate, stir and disperse evenly, and completely disperse into a suspension; superfine the obtained suspension through a homogenizer, and pack the suspension into Put it into four stainless steel containers in a high-performance ball mill, start the ball mill for 5-10 minutes, and make the suspension fine to make it ultra-fine (95% or more to 15μm or less), and make up for the injection that has been coo...
Embodiment 3
[0039] Weigh 14.0g of amoxicillin and 3.5g of potassium clavulanate, put them into a pulverizer and pulverize until more than 90% of the particles have a particle size of ≤15-20μm; take 86mL of injection-grade soybean oil, heat it to 160°C in a container, stir and sterilize it for 80 minutes, then cool To 72°C, add 2g of injection-grade aluminum stearate to 32mL of soybean oil after cooling, heat and stir to disperse evenly, cool to 32°C, filter the oil gel through a 150-mesh metal filter; under stirring (speed: 140r / min) slowly add the crushed amoxicillin and potassium clavulanate, stir and disperse evenly, and completely disperse into a suspension; ultrafine the obtained suspension through a homogenizer, and put the suspension into In the four stainless steel containers in the high-performance ball mill, start the ball mill for 5 to 10 minutes to make the suspension fine to make it ultra-fine (95% reaches 15 μm or less), and make up for the injection cooling to 29±1℃ Soybea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com