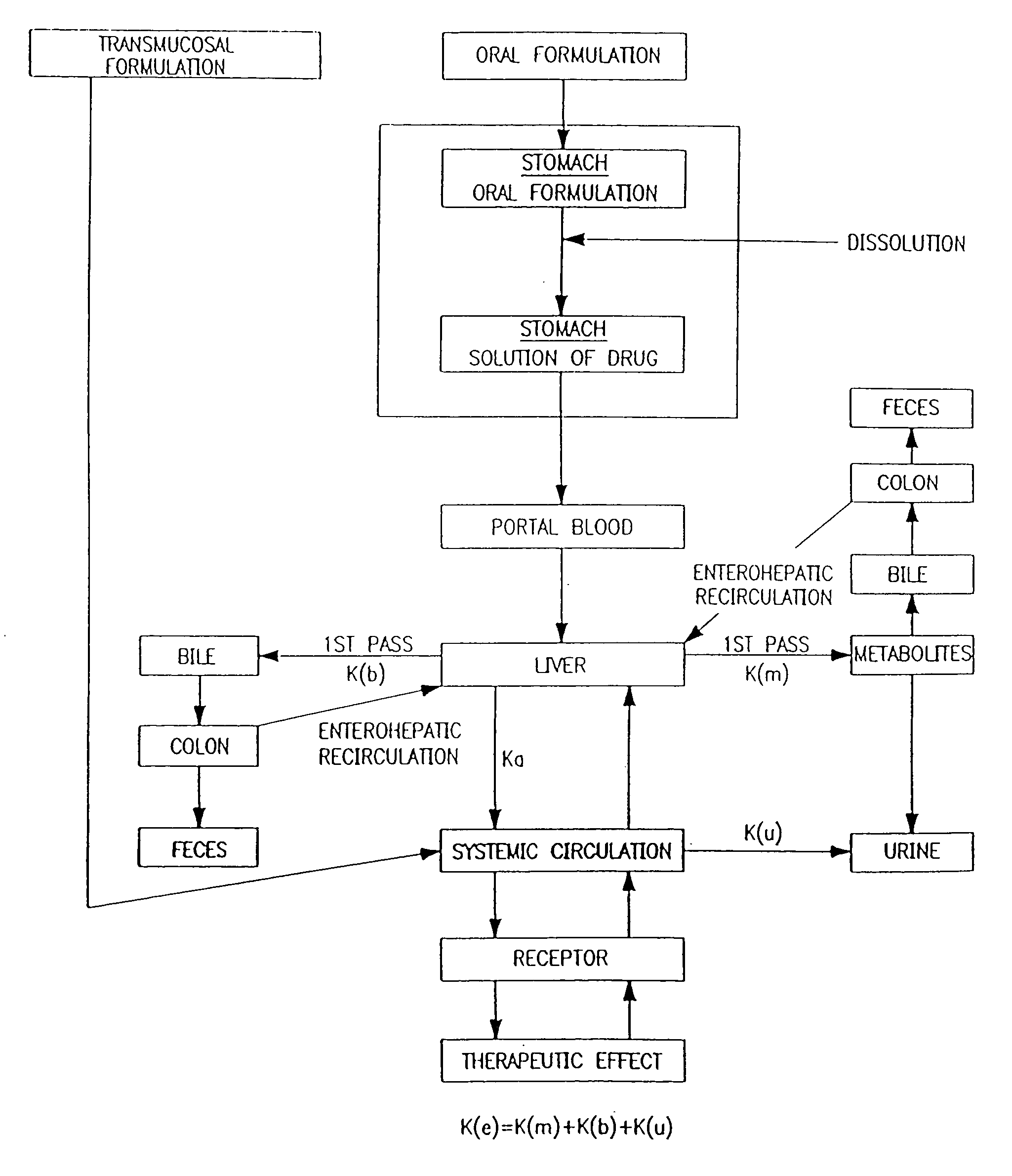
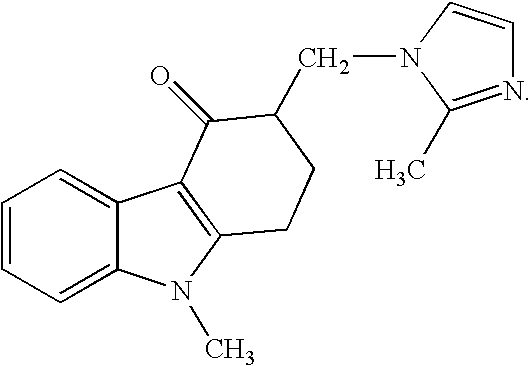
Buccal, polar and non-polar spray containing ondansetron
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Biologically Active Peptides Including Peptide Hormones
[0070] A. Cyclosporine Lingual Spray
preferredmost preferredAmountsamountamountcyclosporine5-5010-3515-25water5-207.5-50 9.5-12 ethanol5-607.5-50 10-20polyethylene glycol20-60 30-4535-40flavors0.1-5 1-42-3
[0071] B. Cyclosporine Non-Polar Lingual Spray
preferredmost preferredAmountsamountamountcyclosporine 1-50 3-40 5-30Migylol202530-40Polyoxyethylated castor oil202530-40Butane25-8030-7033-50flavors0.1-5 1-42-3
[0072] C. Cyclosporine Non-Polar Bite Caosule
preferredmost preferredAmountsamountamountcyclosporine 1-355-2510-20olive oil25-6035-55 30-45polyoxyethylated25-6035-55 30-45oleic glyceridesflavors0.1-5 1-4 2-3
[0073] D. Cyclosporine Bite Capsule
preferredmost preferredAmountsamountamountcyclosporine5-5010-3515-25polyethylene20-60 30-4535-40glycolglycerin5-307.5-25 10-20propylene glycol5-307.5-25 10-20flavors0.1-10 1-83-6
[0074] E. Sermorelin (as the Acetate) Lingual Spray
preferredmostAmountsamountpreferredsermorelin ...
example 2
CNS Active Amines and Their Salts: Including but not Limited to Tricyclic Amines, GABA Analogues, Thiazides, Phenothiazine Derivatives, Serotonin Antagonists and Serotonin Reuptake Inhibitors
[0078] A. Sumatriptan Succinate Lingual Spray
preferredmost preferredAmountsamountamountsumatriptan succinate0.5-30 1-2010-15ethanol5-607.5-5010-20propylene glycol5-307.5-2010-15polyethylene glycol0-60 30-4535-40water5-307.5-2010-15flavors0.1-5 1-42-3
[0079] B. Sumatriptan Succinate Bite Capsule
preferredmost preferredAmountsamountamountsumatriptan succinate0.01-5 0.05-3.5 0.075-1.75 polyethylene glycol25-7030-6035-50glycerin25-7030-6035-50flavors0.1-10 1-83-6
[0080] C. Clozepine Lingual Spray
preferredmost preferredAmountsamountamountclozepine0.5-30 1-2010-15ethanol5-607.5-5010-20propylene glycol5-307.5-2010-15polyethylene glycol0-60 30-4535-40water5-307.5-2010-15flavors0.1-5 1-42-3
[0081] D. Clozepine Non-Polar Lingual Spray with Propellant
preferredmost preferredAmountsamountamo...
example 3
Sulfonylureas
[0085] A. Glyburide Lingual Spray
preferredmost preferredAmountsamountamountglyburide0.25-25 0.5-200.75-15 ethanol5-60−7.5-50 10-20propylene glycol5-307.5-2010-15polyethylene glycol0-60 30-4535-40water2.5-30 5-20 6-15flavors0.1-5 1-42-3
[0086] B. Glyburide Non-Polar Bite Capsule
preferredmost preferredAmountsamountamountglyburide0.01-10 0.025-7.5 0.1-4 olive oil30-6035-55 30-50polyoxyethylated oleic30-6035-55 30-50glyceridesflavors0.1-5 1-4 2-3
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com