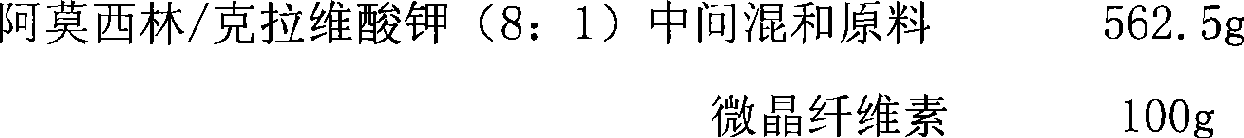Amoxicillin/clavulanate potassium tablet and preparation method thereof
A technology of potassium clavulanate and amoxicillin, which is applied in the field of medicine, can solve unseen problems, and achieve the effects of simple industrial production, good stability and quality controllability, and good safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] prescription:
[0053]
[0054] Coating prescription:
[0055]
[0056] Preparation Process:
[0057] Take the prescription amount and mix amoxicillin / potassium clavulanate (8:1), crystalline mannitol, low-substituted hydroxypropyl methylcellulose, cross-linked polyvinylpyrrolidone, lactose, and magnesium stearate over 80% respectively. Mesh sieve for standby; then take the prescription amount of amoxicillin / potassium clavulanate (8:1) intermediate mixed raw materials, crystalline mannitol, low-substituted hydroxypropyl methylcellulose, cross-linked polyvinylpyrrolidone, lactose in the mixer Take the above mixed powder, place it in a press to granulate by rolling, pass through a 16-mesh sieve to granulate, weigh, mix in the prescribed amount of magnesium stearate, mix evenly, and press it into a tablet containing 500mg of amoxicillin, Clavulanic acid 62.5 mg plain tablets; Opadry is used as the coating material, and the coating solution is prepared with absolute...
Embodiment 2
[0060] prescription:
[0061]
[0062]
[0063] Coating prescription:
[0064]
[0065] Preparation Process:
[0066] Amoxicillin / potassium clavulanate (8:1) intermediate mixed raw materials, compressible starch, low-substituted hydroxypropyl methylcellulose, cross-linked polyvinylpyrrolidone, lactose, and magnesium stearate were mixed separately in the prescribed amount. 80 mesh sieves for subsequent use; then take the intermediate mixed raw materials of amoxicillin / potassium clavulanate (8:1), compressible starch, low-substituted hydroxypropyl methylcellulose, cross-linked polyvinylpyrrolidone, lactose in Mix in a mixer; take the above mixed powder, put it in a press to granulate by rolling, pass through a 16-mesh sieve to granulate, weigh, mix in the prescribed amount of magnesium stearate, mix evenly, and press to make each tablet contain amoxicillin 500mg, clavulanic acid 62.5mg plain tablets; use Opadry as the coating material, and use absolute ethanol to prep...
Embodiment 3
[0069] prescription:
[0070]
[0071] Coating prescription:
[0072]
[0073] Preparation Process:
[0074] Take the prescription amount and pass through 80 meshes of amoxicillin / potassium clavulanate (8:1), compressible starch, low-substituted hydroxypropyl methylcellulose, cross-linked polyvinylpyrrolidone, lactose, and micropowdered silica gel respectively. Sieve for later use; then take the prescription amount of amoxicillin / potassium clavulanate (8:1) intermediate mixed raw materials, compressible starch, low-substituted hydroxypropyl methylcellulose, cross-linked polyvinylpyrrolidone, lactose, micronized silica gel Mix evenly in a mixer; take the above mixed powder, place it in a press for granulation by rolling, pass through a 16-mesh sieve for granulation, weigh, and compress into plain tablets each containing 500 mg of amoxicillin and 62.5 mg of clavulanic acid; Opadry is used as the coating material, and the coating solution is formulated with absolute ethan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com