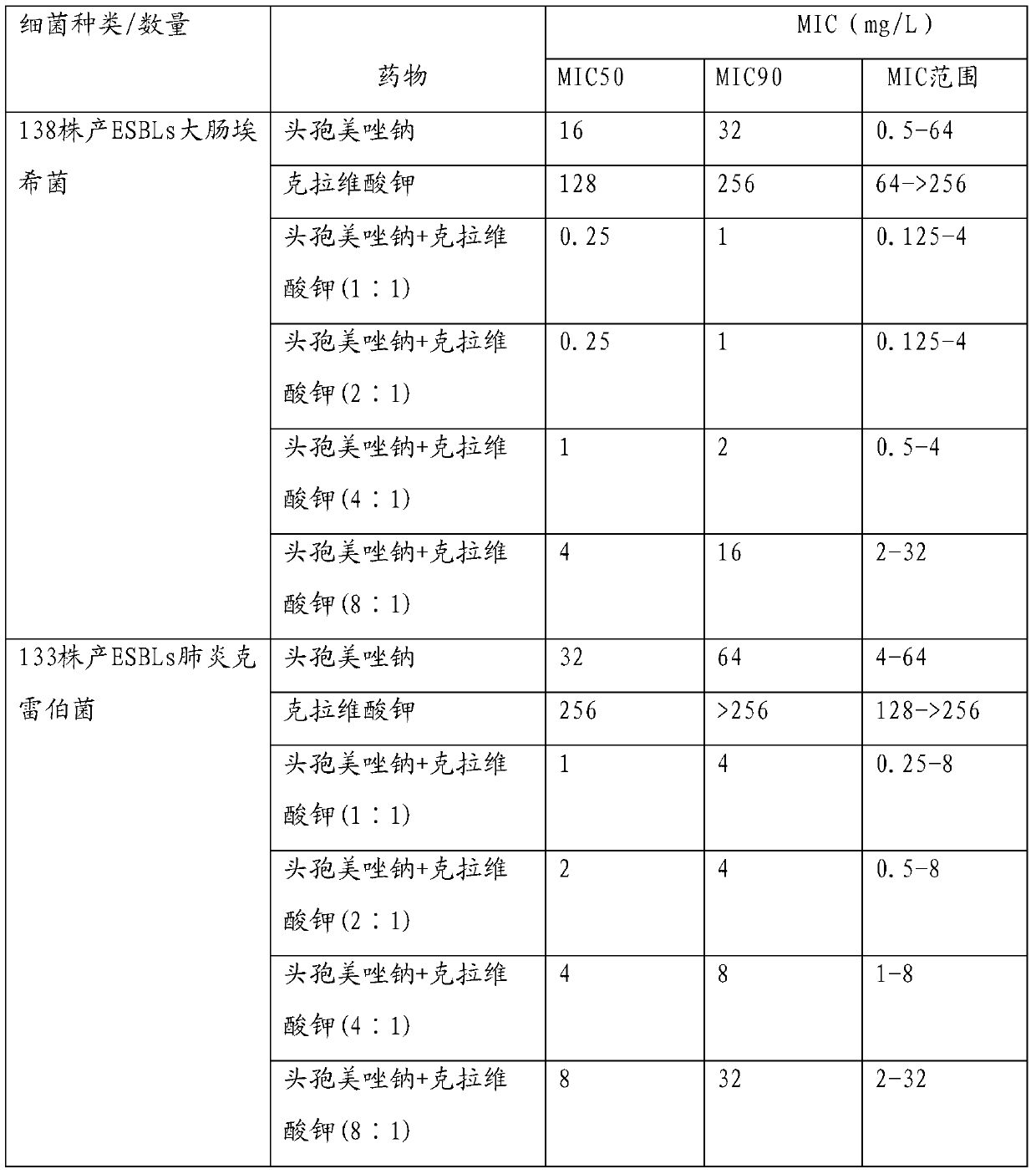
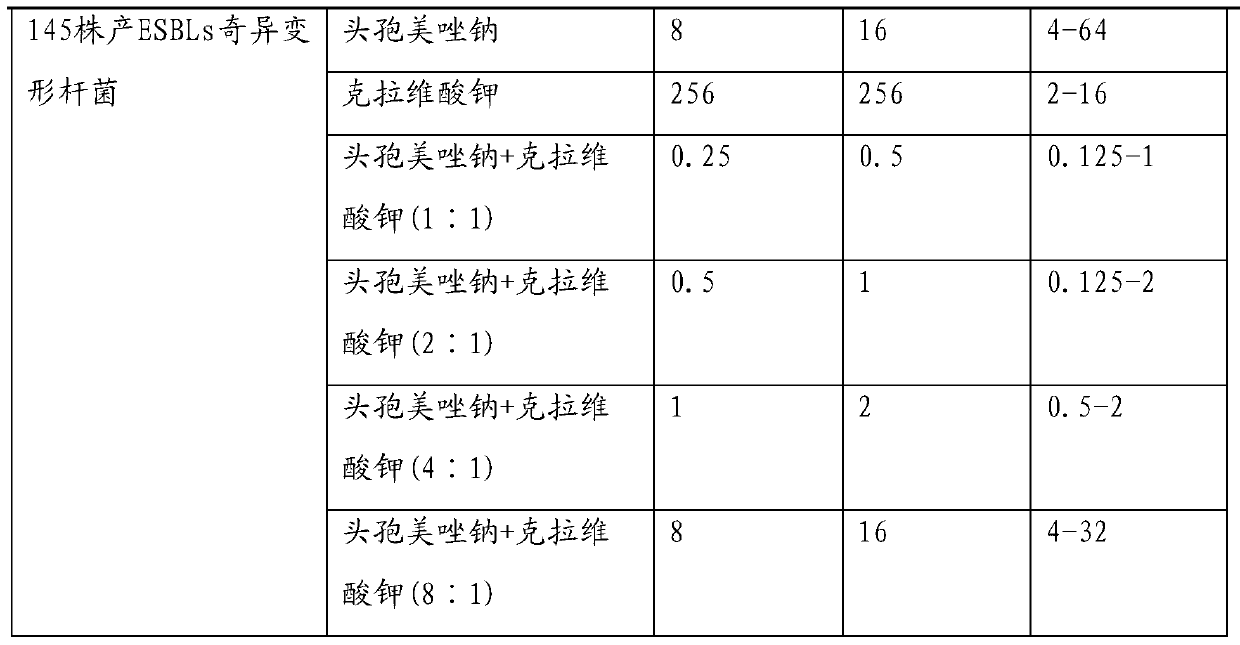
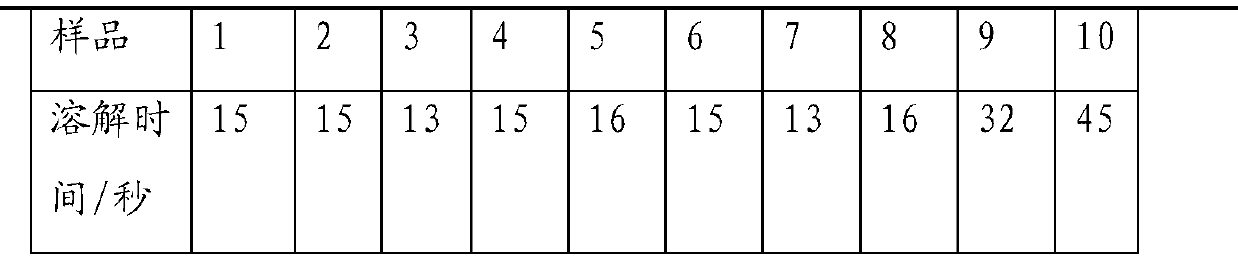
Injection-use pharmaceutical composition comprising cefmetazole sodium and clavulanate potassium
A technology of azolium sodium clavulanate potassium and cefmetazole sodium, which is applied in the field of pharmaceutical compositions of cefmetazole sodium and clavulanate potassium for injection, which can solve the problem that the antibacterial activity is greatly affected and the optimization degree of antibacterial activity cannot be expected , Inhibition enzyme spectrum and stability differences, etc., to achieve high antibacterial activity, improve drug stability, and excellent stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Cefmetazole Sodium 100g
[0030] Potassium clavulanate 100g
[0031] L-Arginine 20g
[0032] n-Butyl cyanoacrylate 200g
[0033] Tween 80 100g
[0034] PVP 60g
[0035] Mannitol 50g
[0036] Glycine 50g
[0037] A total of 500 sticks were prepared
[0038] Add cefmetazole sodium, L-arginine and potassium clavulanate to 2.5L water for injection in turn, stir and dissolve at room temperature, add alkyl cyanoacrylate monomer, Tween and PVP, continue stirring for 3 hours, add mannitol and glycine , add 0.5L of water for injection, stir until completely dissolved, adjust the pH to 5-7, filter aseptically with a 0.22 μm filter membrane; divide the filtrate into sterilized vials, pre-freeze at -45°C for 2 hours, Freeze-dried with a freeze dryer, sealed with a sterile rubber stopper, and then sealed with a sterile aluminum cap. Example 2
Embodiment 2
[0039] Cefmetazole Sodium 150g
[0040] Potassium clavulanate 100g
[0041] L-Arginine 20g
[0042] n-Butyl cyanoacrylate 300g
[0043] Tween 80 100g
[0044] PVP 50g
[0045] Mannitol 50g
[0046] Glycine 50g
[0047] A total of 500 sticks were prepared
[0048] Add cefmetazole sodium, L-arginine and potassium clavulanate to 2.5L water for injection in turn, stir and dissolve at room temperature, add alkyl cyanoacrylate monomer, Tween and PVP, continue stirring for 3 hours, add mannitol and glycine , add 0.5L of water for injection, stir until completely dissolved, adjust the pH to 5-7, filter aseptically with a 0.22 μm filter membrane; divide the filtrate into sterilized vials, pre-freeze at -45°C for 2 hours, Freeze-dried with a freeze dryer, sealed with a sterile rubber stopper, and then sealed with a sterile aluminum cap. Example 3
Embodiment 3
[0049] Cefmetazole Sodium 200g
[0050] Potassium clavulanate 100g
[0051] L-Arginine 40g
[0052] Isobutyl cyanoacrylate 400g
[0053] Tween 20 100g
[0054] PVP 60g
[0055] Mannitol 55g
[0056] Glycine 55g
[0057] A total of 500 sticks were prepared
[0058] Add cefmetazole sodium, L-arginine and potassium clavulanate to 2.5L water for injection in turn, stir and dissolve at room temperature, add alkyl cyanoacrylate monomer, Tween and PVP, continue stirring for 3 hours, add mannitol and glycine , add 1L of water for injection, stir until completely dissolved, adjust the pH to 5-7, filter aseptically with a 0.22μm filter membrane; divide the filtrate into sterilized vials, pre-freeze at -45°C for 2 hours, and use Freeze-dried in a freeze dryer, sealed with a sterile rubber stopper, and then sealed with a sterile aluminum cap. Example 4
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com