Cefaclor granule pharmaceutical composition
A technology of Clow granule and composition, which is applied in the field of cefaclor granule pharmaceutical composition, which can solve the problems of short total length of digestive tract, decreased bioavailability, decreased drug efficacy, etc., and achieves reduced drug burden and good dissolution stability , the effect of alleviating the pressure of medical insurance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
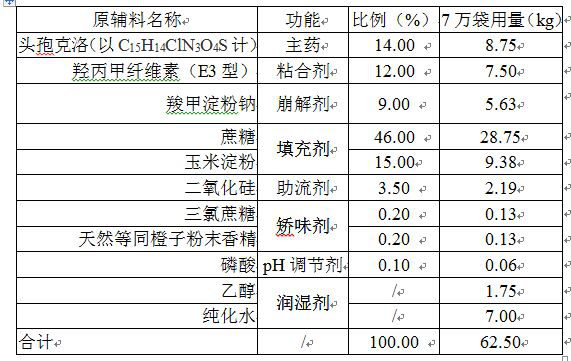
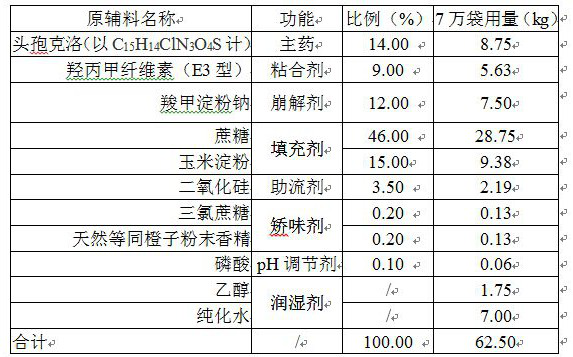
[0037] prescription:
[0038] .
[0039] Preparation:
[0040] Step 1. Add the prescribed amount of ethanol and phosphoric acid into the prescribed amount of purified water under stirring, and stir evenly.
[0041] Step 2. Add the prescribed amount of cefaclor, sucrose, corn starch, hypromellose, sodium carboxymethyl starch, silicon dioxide, sucralose and natural equivalent orange powder flavor into the wet granulator, and mix well.
[0042] Step 3. Under stirring, add the material obtained in step 1 to the granulator with the material in step 2 in the form of spraying, requiring rapid spraying (spraying pressure is greater than 0.3Mpa, completed within one minute), after spraying , continue to wet mix soft materials.
[0043] Step 4. Wet the soft material prepared in step 3 using a swaying granulation mechanism, and use a sieve with a mesh number of 20 mesh.
[0044] Step 5. Transfer the wet granules obtained in step 4 to the boiling dryer, set the material temperature ...
Embodiment 2
[0049] prescription:
[0050] .
[0051] Preparation method: Steps 1 to 8 are the same as in Example 1, and 65065 bags of finished products are obtained, with a yield of 92.95%.
Embodiment 3
[0053] prescription:
[0054] .
[0055] Preparation:
[0056] Step 1 to step 8 are the same as in Example 1, and 64758 bags of finished products are obtained, with a yield of 92.51%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com