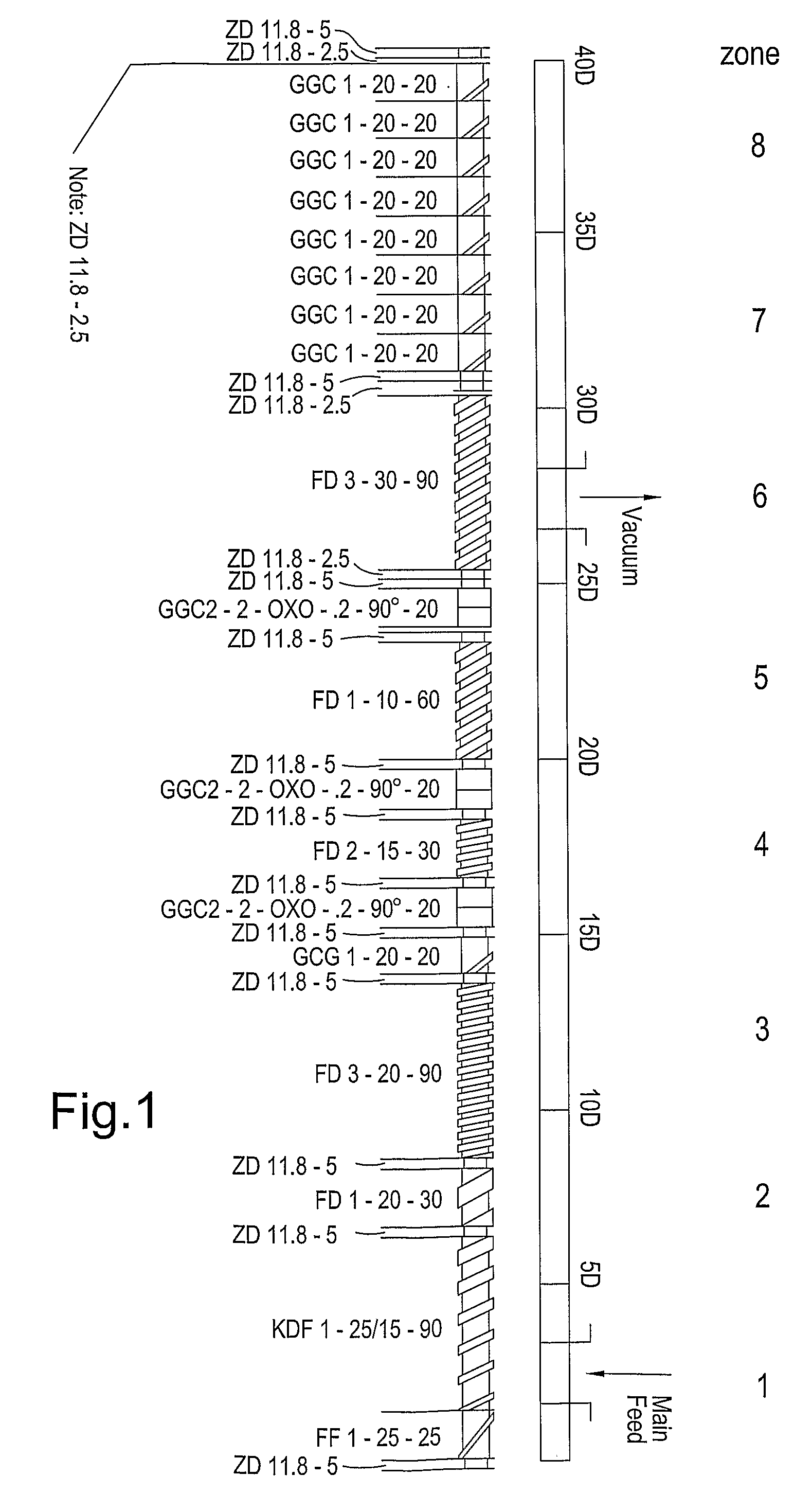
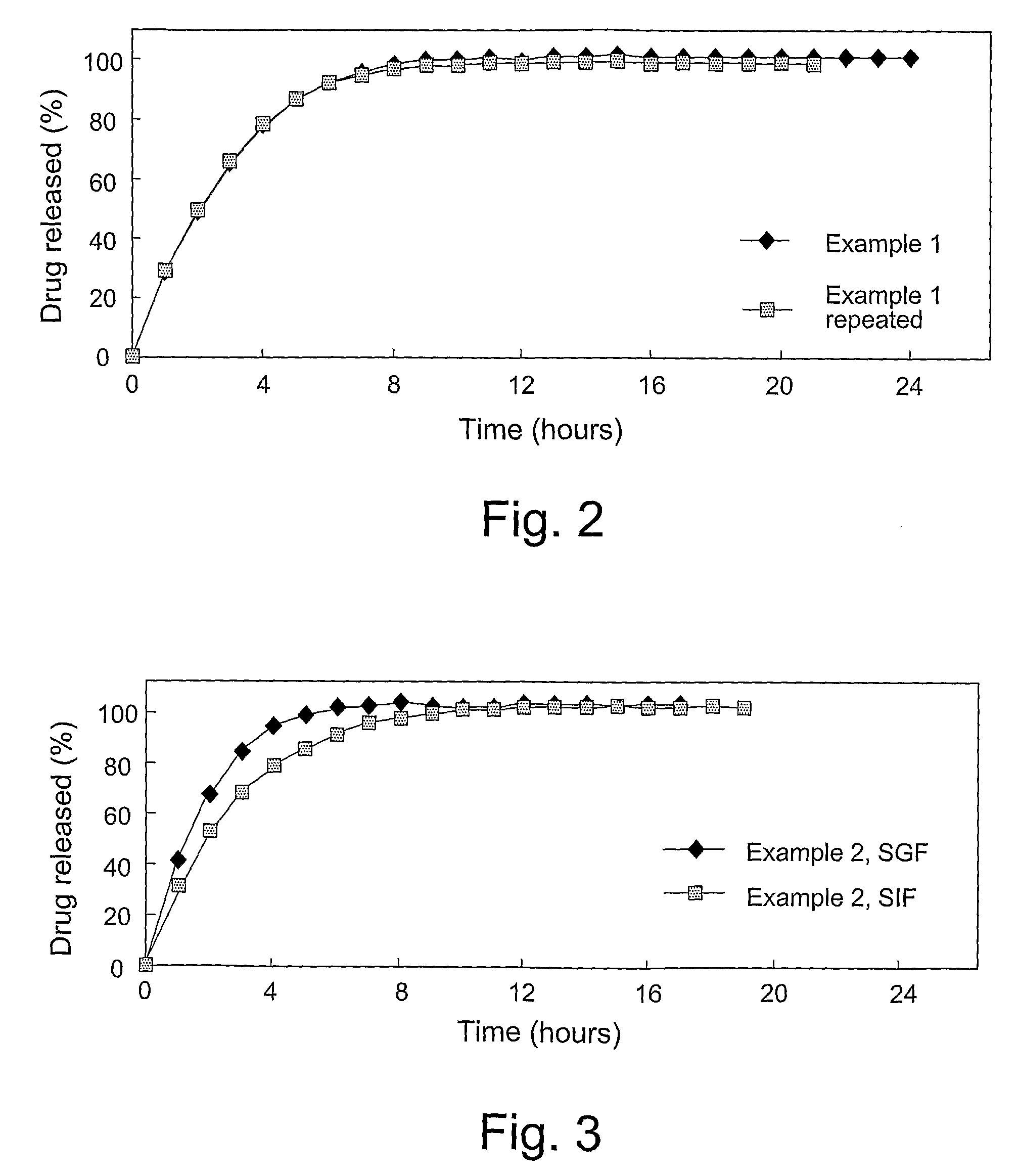
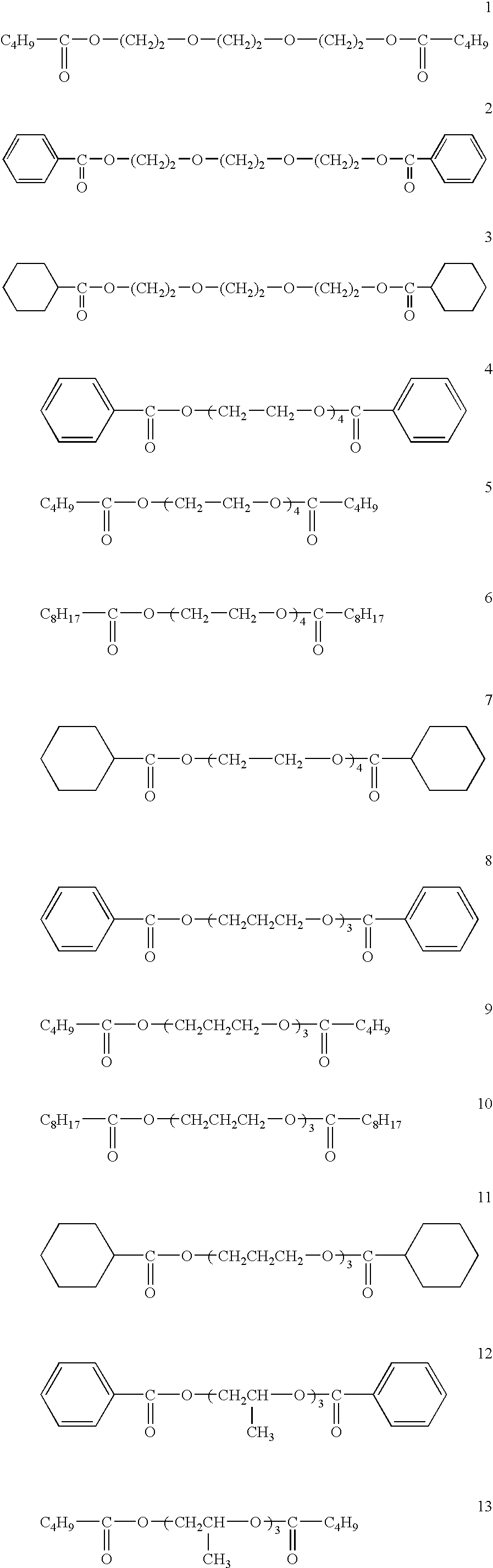
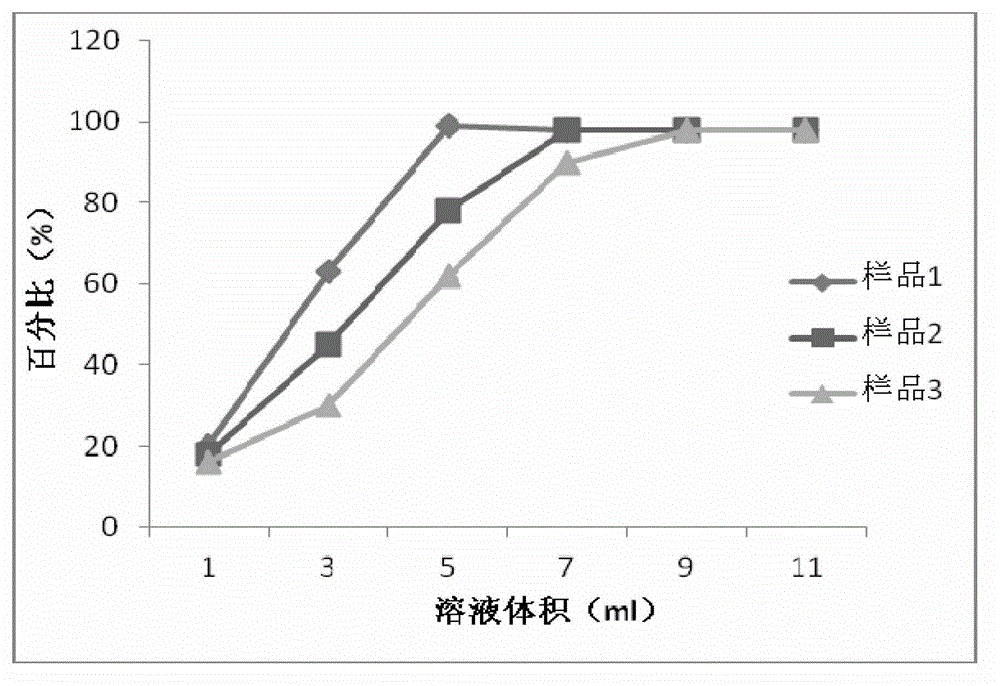
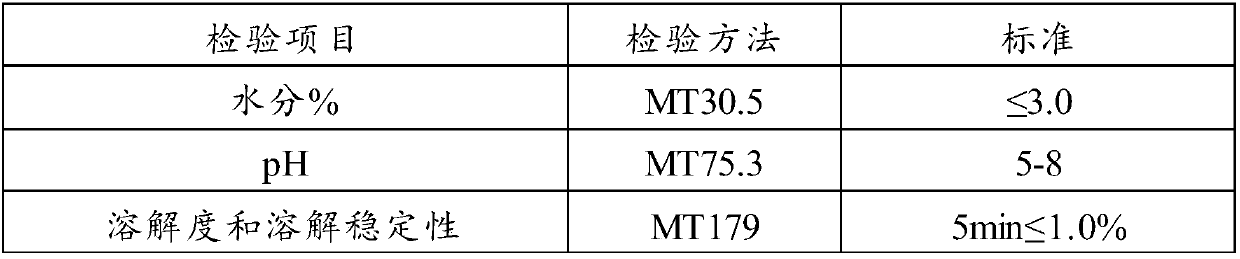
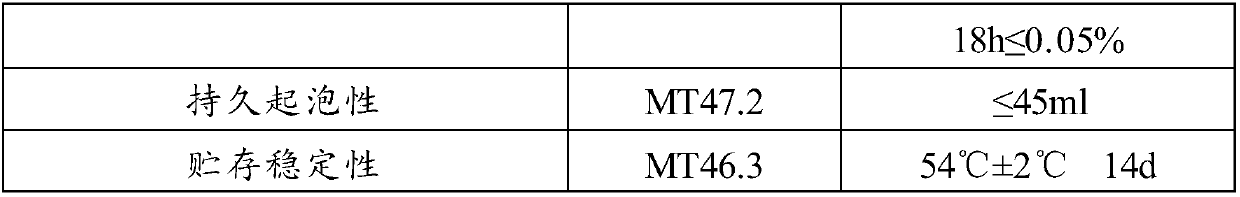
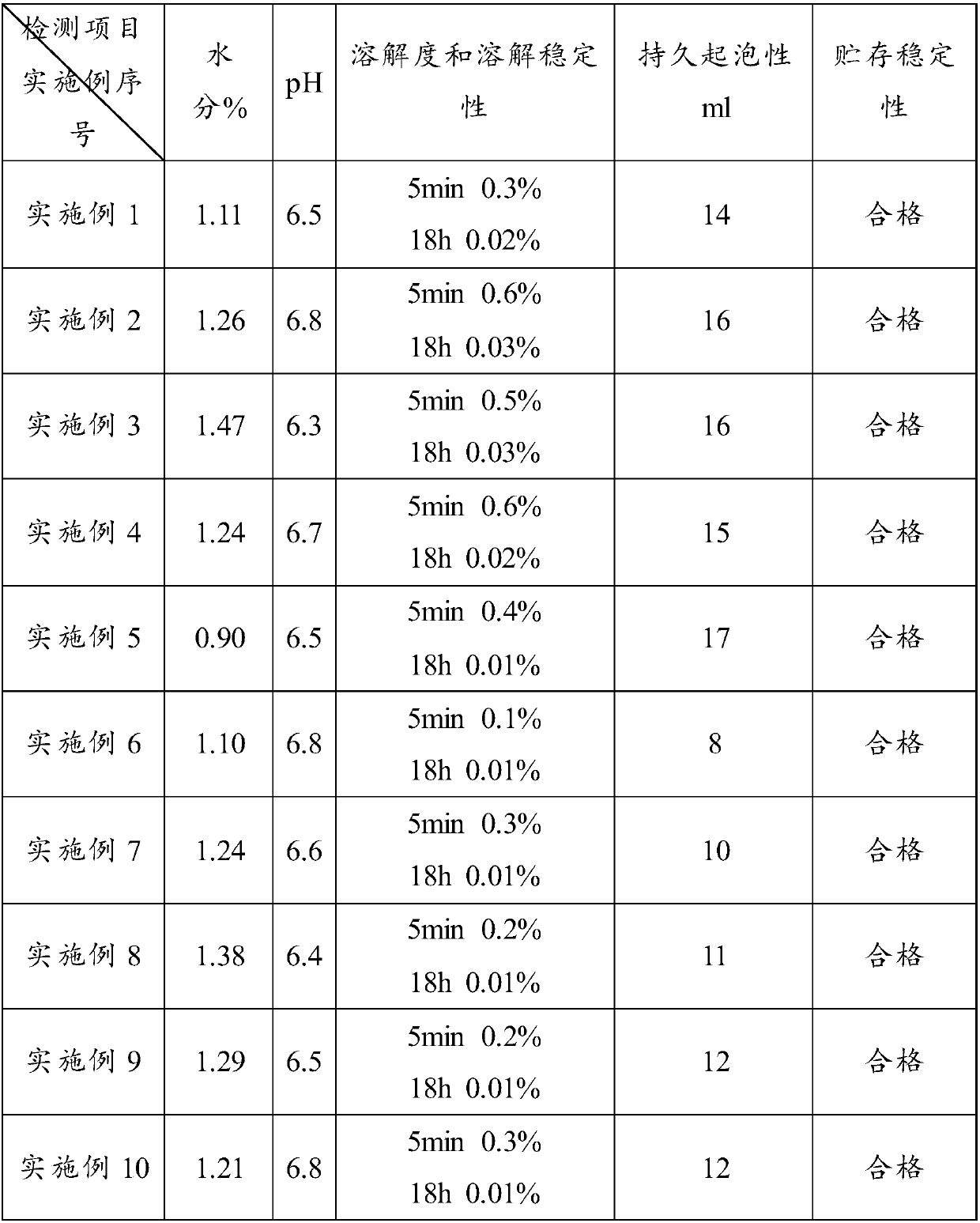
Patents
Literature
81results about How to "Good dissolution stability" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
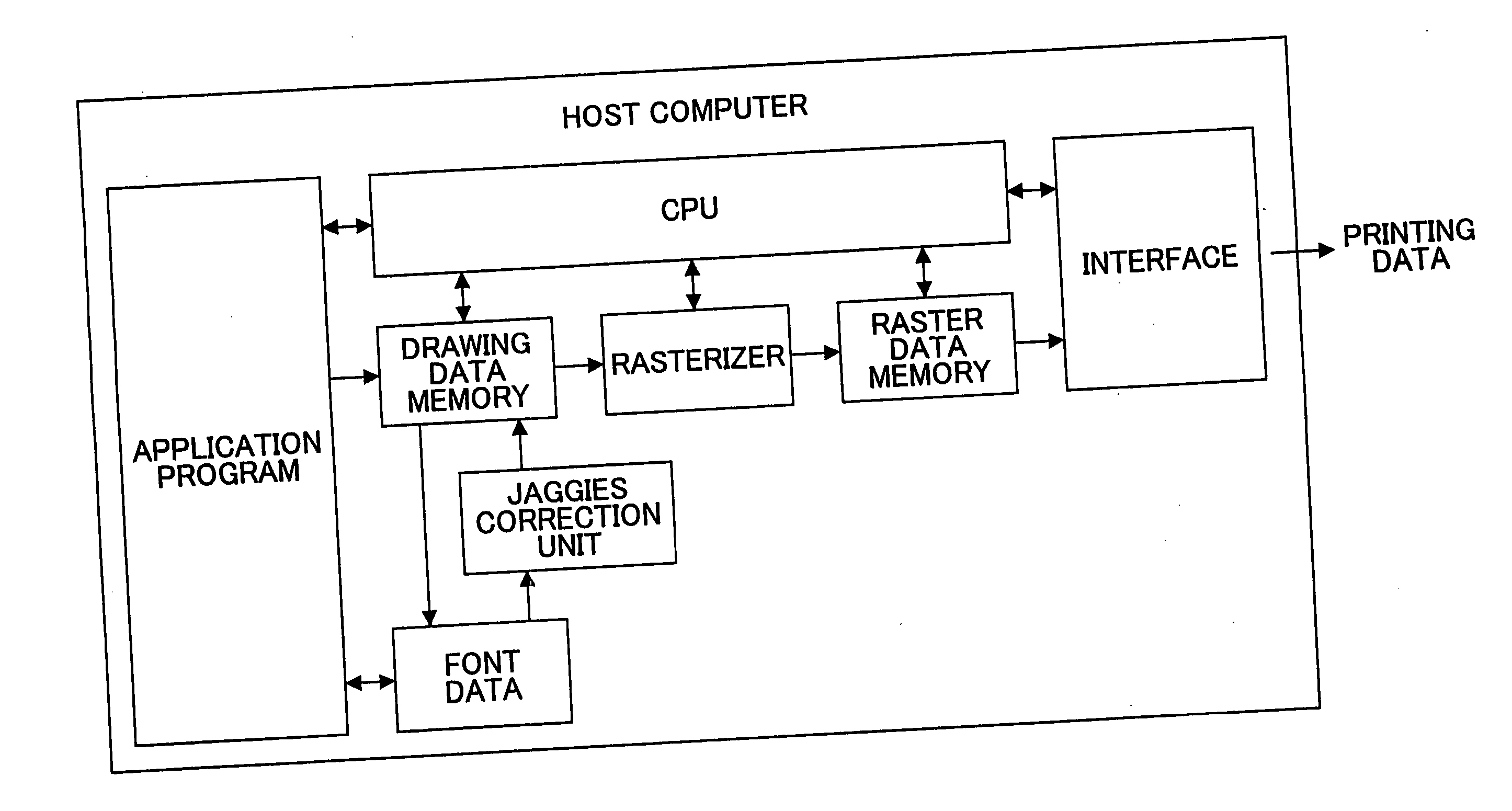
Image-processing method and apparatus, and image-forming apparatus
InactiveUS20060098232A1Reduce step change partImprove image qualityImage enhancementDigitally marking record carriersHueGraphics
In an image-processing method and apparatus, dots forming a step-change part of an outline of an image of characters and / or graphics which image is subjected to half-tone processing are detected, and dots surrounding the detected dots forming the step-change part are transformed into respective dot data each having a size that is smaller than a size of the detected dots forming the step-change part. In the image-processing method and apparatus, one of different transformation methods is selected according to an inclination of the outline in order to produce the dot data in the transforming.
Owner:RICOH KK
Multiparticulates
InactiveUS20080260815A1Reduce adhesionReduce the required powerOrganic active ingredientsPowder deliveryActive agentExcipient
Extrusion of a mix containing a pharmaceutically active agent can be achieved using a plasticising excipient in an amount sufficient to act as plasticiser and also act as lubricant, thereby avoiding the need for inclusion of a lubricant. The invention provides multiparticulates with controlled release properties, substantially free of lubricant. The present invention is preferably directed to extruded multiparticulates containing an opioid such as oxycodone, an ammonium methacrylate copolymer such as Eudragit® RSPO, a plasticising excipient such as preferably stearyl alcohol and a water permeability modifier such as preferably Eudragit® RLPO. The obtained multiparticulates show a release rate profile which is pH-independent.
Owner:EURO-CELTIQUE SA
Optical film, polarizing plate and method for forming optical film
InactiveUS20060105155A1Reduce harmful effectsReduce environmental loadSynthetic resin layered productsCellulosic plastic layered productsCelluloseOrganic solvent
An optical film comprises a support having thereon a coat layer formed by directly coating a coating solution containing at least one organic solvent selected from ketones and esters on the support surface, the support comprising a cellulose acylate film containing at least one plasticizer, wherein a surface plasticizer amount in the cellulose acylate film is from 1 to 20 mass % of the cellulose acylate film.
Owner:FUJIFILM CORP
Lansoprazole crystalline compound, enteric capsule thereof and preparation method of Lansoprazole crystalline compound
ActiveCN102558154AImprove medication safetyImprove stabilityOrganic active ingredientsOrganic chemistryLansoprazoleCrospovidones
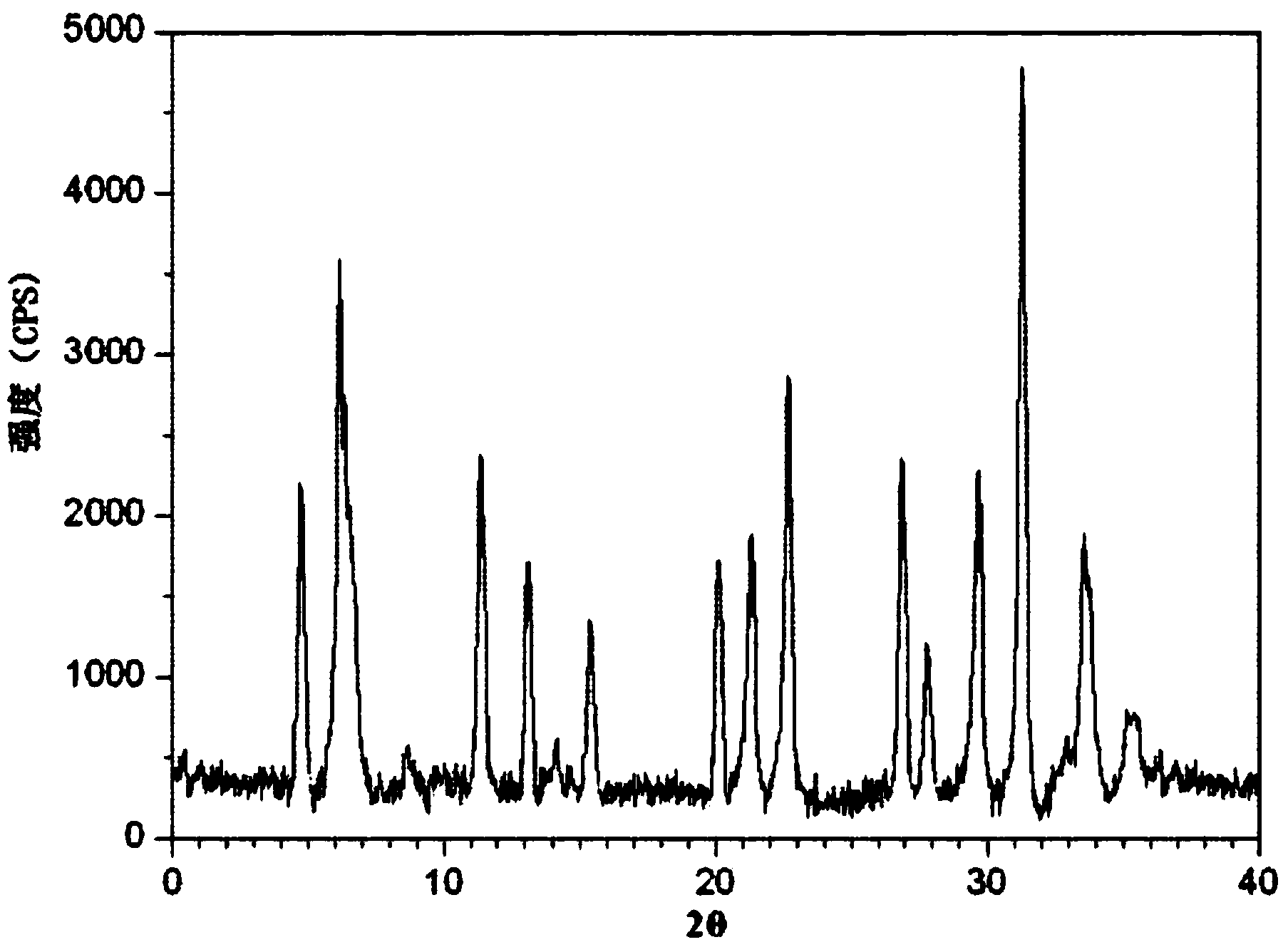
The invention relates to a lansoprazole crystalline compound. An X-ray powder diffraction pattern represented by a diffraction angle of 2 theta + / - 0.2 DEG displays feature diffraction peaks at the positions of 5.8 DEG, 7.5 DEG, 9.1 DEG, 11.8 DEG, 12.1 DEG, 12.8 DEG, 13.3 DEG, 15.6 DEG, 16.7 DEG, 18.3 DEG, 20.4 DEG, 25.7 DEG, 26.8 DEG and 31.5 DEG. The invention also relates to a lansoprazole enteric capsule containing the lansoprazole crystalline compound. The lansoprazole enteric capsule comprises 20 to 60 parts of l crystalline compound, 90 to 140 parts of microcrystalline cellulose, 1.5 to 3.5 parts of disodium hydrogen phosphate, 2 to 5 parts of anhydrous sodium sulphite, 1 to 10 parts of crospovidone, 0.8 to 4.2 parts of lauryl sodium sulfate, 2 to 8 parts of povidone K30 and 1 to 3 parts of magnesium stearate.
Owner:HAINAN JINRUI PHARMA CO LTD
Cefoxitin sodium crystal compound and cefoxitin sodium composition powder injection
ActiveCN102358744AImprove solubilitySolubility stabilityAntibacterial agentsPowder deliveryCefoxitin SodiumSodium benzoate
The invention relates to a cefoxitin sodium crystal compound. The method of powder X-ray diffractometry is utilized to determine the cefoxitin sodium crystal compound, and an X-ray powder diffraction pattern represented by the diffraction angle of 2theta+-0.2 degrees has characteristic diffraction peaks at 5.3 degrees, 8.6 degrees, 11.9 degrees, 13.2 degrees, 15.5 degrees, 16.1 degrees, 19.0 degrees, 22.3 degrees and 24.4 degrees. The invention also relates to a cefoxitin sodium composition powder injection containing the cefoxitin sodium crystal compound, and the cefoxitin sodium composition powder injection comprises 95 to 100 parts of the cefoxitin sodium crystal compound and 0.1 to 1 part of sodium benzoate.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +2
A kind of cefminox sodium crystalline compound and its composition powder injection
ActiveCN102276630AImprove solubilitySolubility stabilityAntibacterial agentsPowder deliveryCEFMINOX SODIUMX-ray
The invention relates to a cefminox sodium crystalline compound. The cefminox sodium crystalline compound is determined by a powder X-ray diffraction determination method; and characteristic diffraction peaks are displayed at 5.1, 6.9, 8.5, 10.3, 12.1, 15.1, 15.9, 17.4, 19.5, 21.7 and 24.6 degrees in an X-ray powder diffraction pattern represented by a diffraction angle of 2 theta+ / -0.2 degree. The invention also relates to cefminox sodium composition powder injection containing the cefminox sodium crystalline compound. The composition powder injection comprises the following components: 95 to 100 parts of cefminox sodium crystalline compound and 0.1 to 1 part of sodium benzoate.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Radiation-sensitive composition, multilayer body and method for producing same, and electronic component
ActiveCN1957299AGood dissolution stabilityEfficient coatingSemiconductor/solid-state device manufacturingPhotosensitive materials for photomechanical apparatusEtherSolvent
Disclosed is a very safe radiation-sensitive composition having excellent electrical characteristics and good solution stability which is able to form a coating free from unevenness. Also disclosed are a multilayer body wherein a resin film is formed on a substrate by using this radiation-sensitive composition, and a method for producing such a multilayer body. A radiation-sensitive composition containing a cyclic olefin polymer having a polar protic group, a radiation-sensitive compound, a crosslinking agent and a solvent is characterized in that the solvent contains a dialkylene glycol dialkyl ether having two different alkyl groups in one molecule. A multilayer body comprises a substrate and a resin film formed on the substrate using the above-described radiation-sensitive composition.
Owner:ZEON CORP
Production process of instant maize germ powder
The invention relates to a production process of instant maize germ powder. The instant maize germ powder is prepared through raw material preprocessing, beating, enzymolysis, enzyme inactivation, colloid milling, sterilization, high-pressure homogenization and spray drying by taking maize germ meal as a raw material; the enzymolysis is carried out by adding medium-temperature alpha-amylase accounting for 0.2%-0.4% of the dry weight of the maize germ meal, and the starch hydrolysis DE value (Dextrose Equivalent value) of maize germ meal slurry which is subjected to enzymolysis is controlled between 18% and 22%; the prepared homogenized maize germ meal slurry achieves the easiness for the spray drying, and a finished product does not absorb moisture, so that the dissolution stability of the maize germ powder is enhanced, the deposition has small possibility of appearing in the dissolving process, and the maize germ powder is not caked and agglomerated when being dissolved in water; and the additional value of maize germ meal is increased. The instant maize germ powder obtained through the method disclosed by the invention achieves the protein content more than 23%, is in a faint yellow color and is fast in dissolution; and the dissolved instant maize germ powder is uniform in state and achieves the product dissolution time less than 27 seconds without any unpleasant odor.
Owner:鲁洲生物科技(辽宁)有限公司 +2
Multiparticulates
InactiveUS9259872B2Reduce adhesionReduce the required powerOrganic active ingredientsCosmetic preparationsActive agentExcipient
Extrusion of a mix containing a pharmaceutically active agent can be achieved using a plasticizing excipient in an amount sufficient to act as plasticizer and also act as lubricant, thereby avoiding the need for inclusion of a lubricant. The invention provides multiparticulates with controlled release properties, substantially free of lubricant. The present invention is preferably directed to extruded multiparticulates containing an opioid such as oxycodone, an ammonium methacrylate copolymer such as Eudragit® RSPO, a plasticizing excipient such as preferably stearyl alcohol and a water permeability modifier such as preferably Eudragit® RLPO. The obtained multiparticulates show a release rate profile which is pH-independent.
Owner:EURO-CELTIQUE SA
Enhanced Turndown Process for a Bitumen Froth Treatment Operation
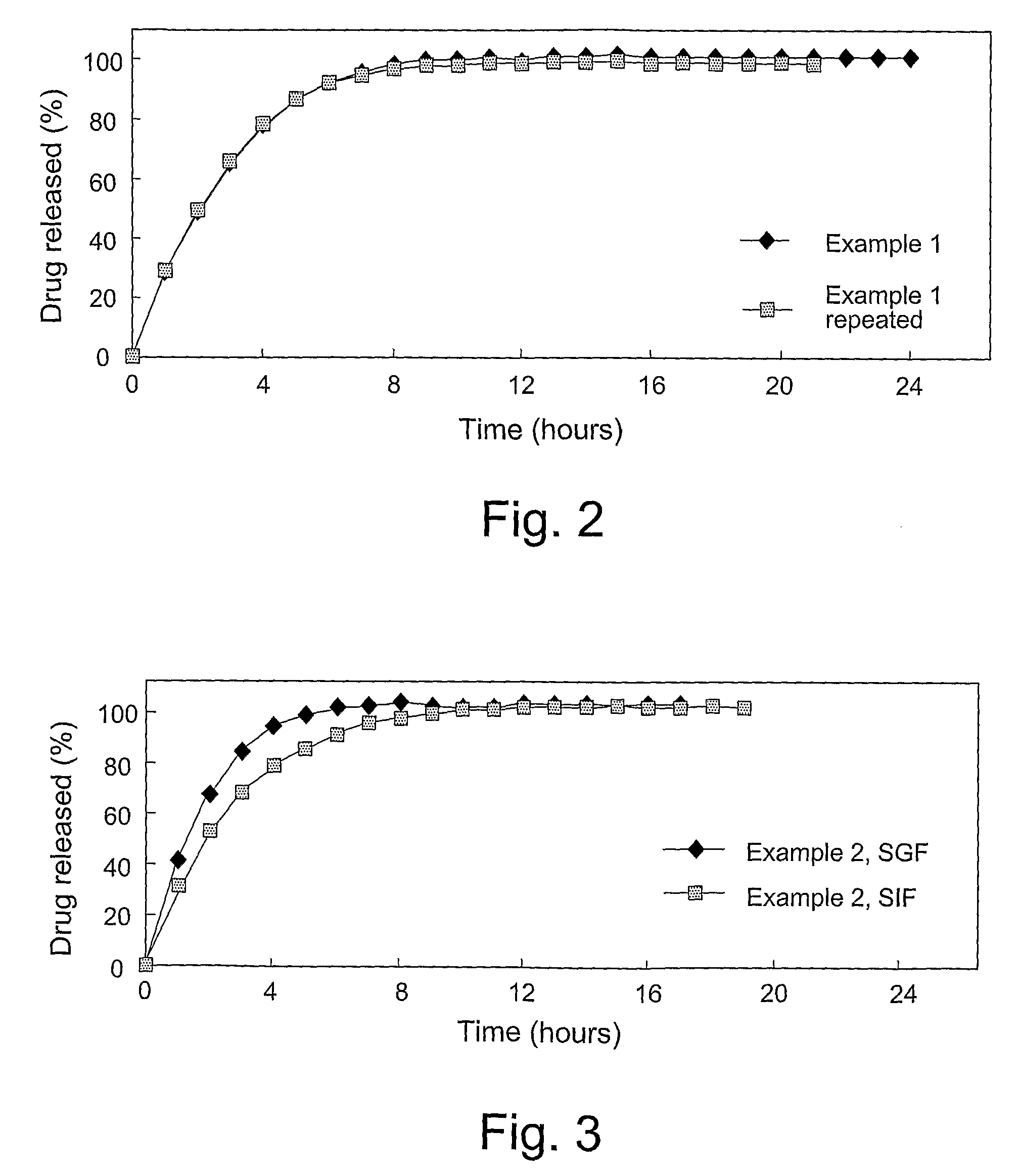
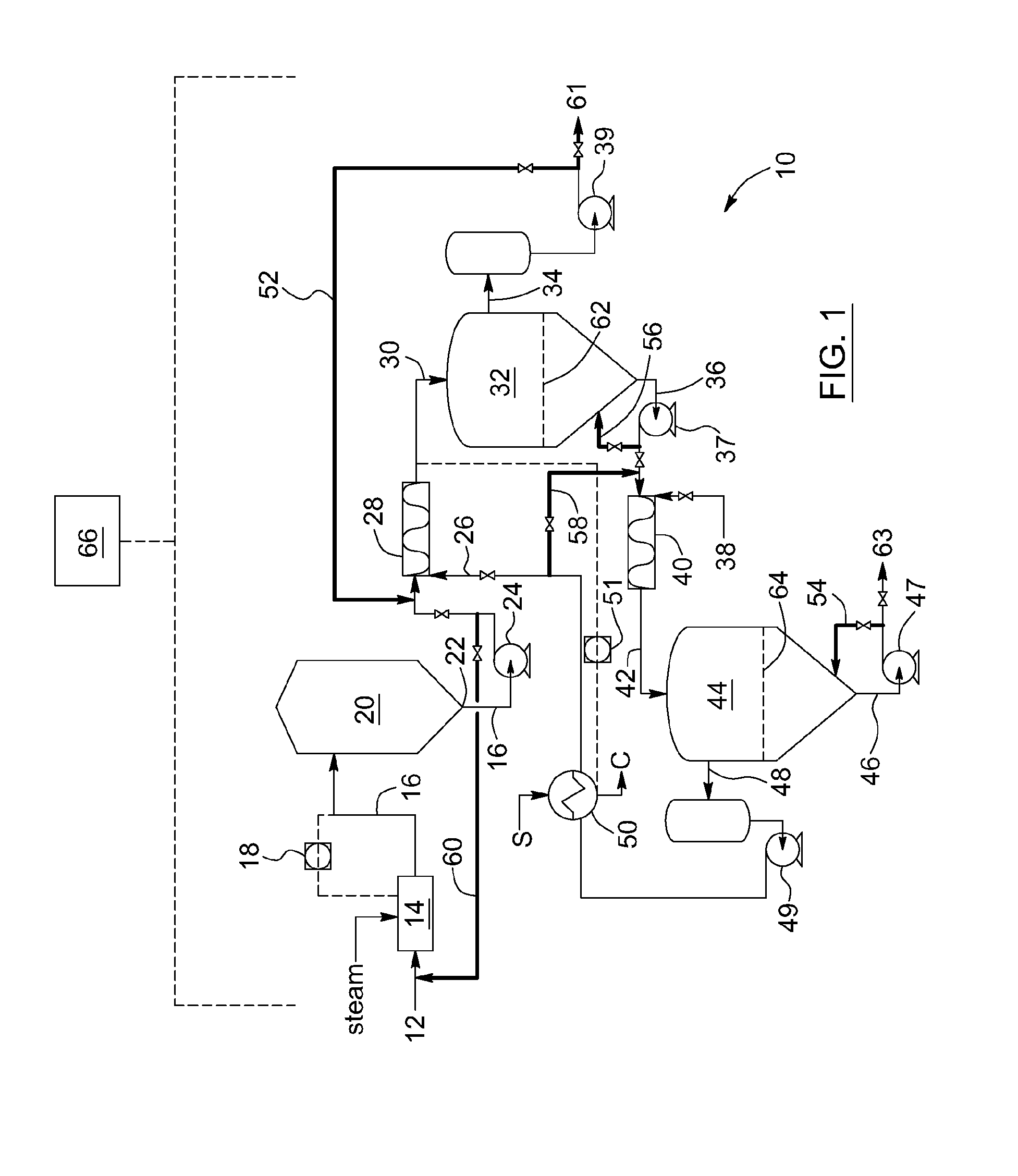
ActiveUS20140048450A1Good dissolution stabilityReduce rateLiquid hydrocarbon mixture productionHydrocarbon oils treatmentThermodynamicsFroth treatment
A process for operating a bitumen froth treatment operation in turndown mode includes adding solvent to bitumen froth to produce diluted bitumen froth and separating it into diluted bitumen and solvent diluted tailings and in response to a reduction in bitumen froth flow recirculating part of the diluted bitumen into the bitumen froth and returning part of the solvent diluted tailings into the step of separating. A method for turndown of separation vessel for PFT includes sustaining the feed flow to vessel; maintaining solvent-to-bitumen ratio in the diluted bitumen froth; and retaining water, minerals and asphaltenes in a lower section of the vessel while sustaining an outlet flow. The use of diluted bitumen derived from PFT as a viscosity modifying agent of the bitumen froth and an associated process are also provided.
Owner:TRUENORTH ENERGY CORP
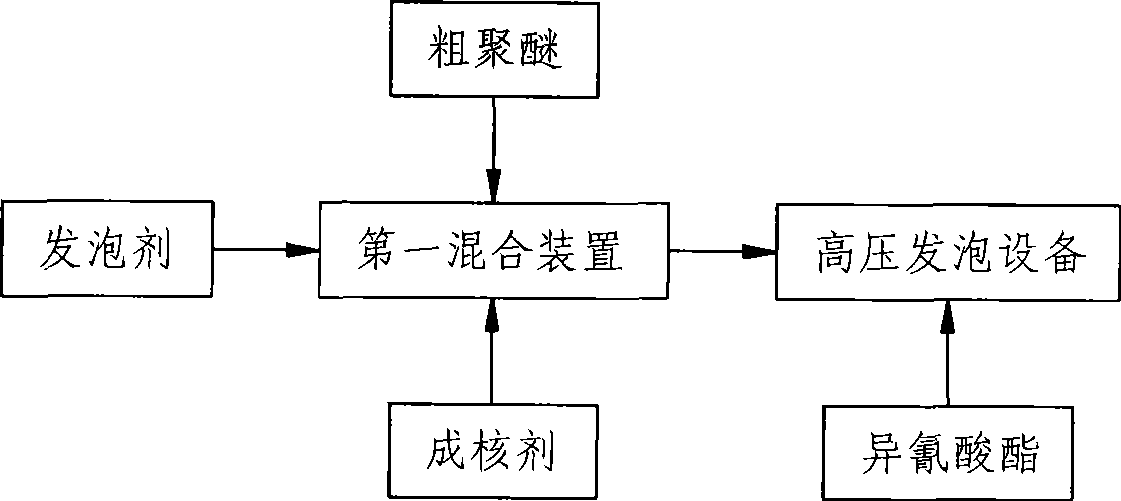
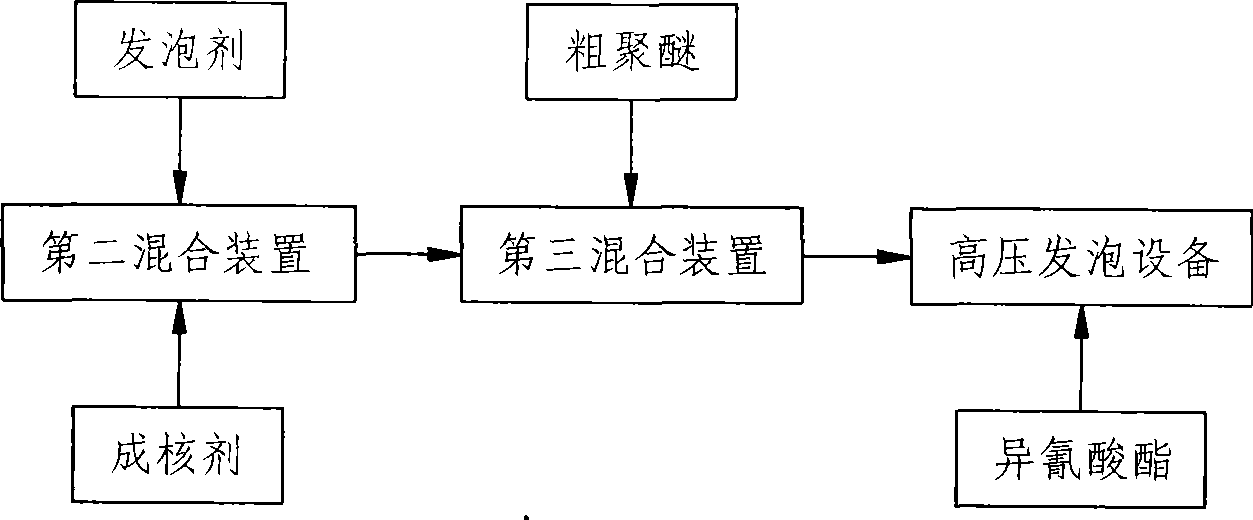
Rigid polyurethane foam and preparation method thereof
InactiveCN101544737AMeet environmental protection requirementsGood dissolution stabilityEpoxySucrose
The invention relates to rigid polyurethane foam and a preparation method thereof. The rigid polyurethane foam is characterized by being formed by the reaction of isocyanates with crude polyether, nucleating agent and vesicant are added during the reaction, the crude polyether is formed by polyether glycol, water, catalyst and stabilizing agent, the polyether glycol is formed by mixing various monomer polyethers, the average hydroxyl value of the polyether glycol is 300-700mg KOH / g, the hydroxyl radial functionality of the polyether glycol is 2-8, each monomer polyether is generated by the reaction of epoxy ethane and / or propylene oxide with evocating agent with 2-8 reactive hydrogen atoms, the evocating agent comprises polyalcohol and polyamine, wherein the polyalcohol comprises glycerin, trimethylolpropane, triethanolamine, pentaerythritol, sorbierite and sucrose alcohol. The rigid polyurethane foam fully accords with the requirement of protecting environment and the polyether glycol having good dissolving stability.
Owner:GUANGDONG GALANZ GRP CO LTD
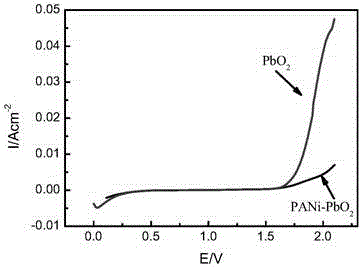
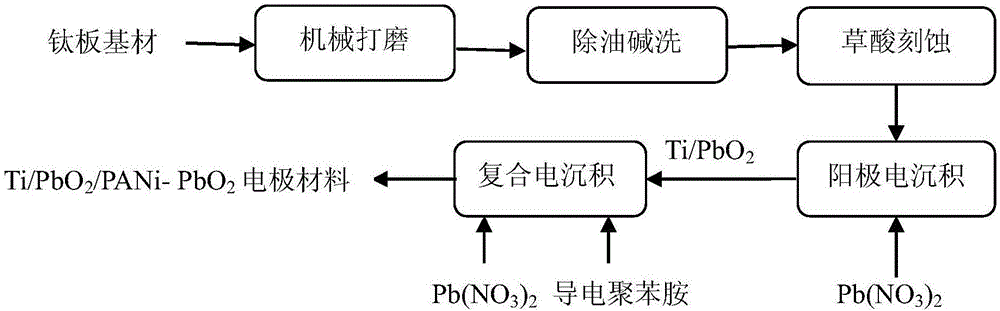
Preparation method titanium-based polyaniline-doped lead dioxide composite electrode material
InactiveCN106044963ADoes not involve polymerizationAvoid uncertaintyWater treatment parameter controlWater contaminantsLead dioxideConductive polymer
The invention relates to a preparation method of a titanium-based polyaniline-doped lead dioxide composite electrode material. The preparation method includes: pretreating a titanium substrate, preparing a PbO2 interlayer and preparing a polyaniline-doped PbO2 surface active layer, to be more specific, dispersing conductive polyaniline particles into an electrodeposition solution, using a composite electrodeposition method to evenly co-deposit polyaniline and PbO2 onto Ti / PbO2, and controlling conditions such as polyaniline use amount, deposition current density and deposition temperature and time to obtain the Ti / PbO2 / PANi-PbO2 composite electrode material with a compact and even surface and evidently refined grains. The preparation method has the advantages that the adverse effect of non-conductive polymer doping on PbO2 conductivity is overcome, the use of polyaniline monomer is avoided, many uncertainties of the doping using the monomer electropolymerization reaction are avoided, and the performance stability of the polyaniline-doped PbO2 electrode material is guaranteed; the obtained electrode material is high in activity and stability, the dissolving stability of the obtained electrode material is evidently better than an undoped PbO2 electrode, and application safety of the PbO2 electrode in the electrooxidation treatment of non-biodegradable organic wastewater.
Owner:XI'AN UNIVERSITY OF ARCHITECTURE AND TECHNOLOGY
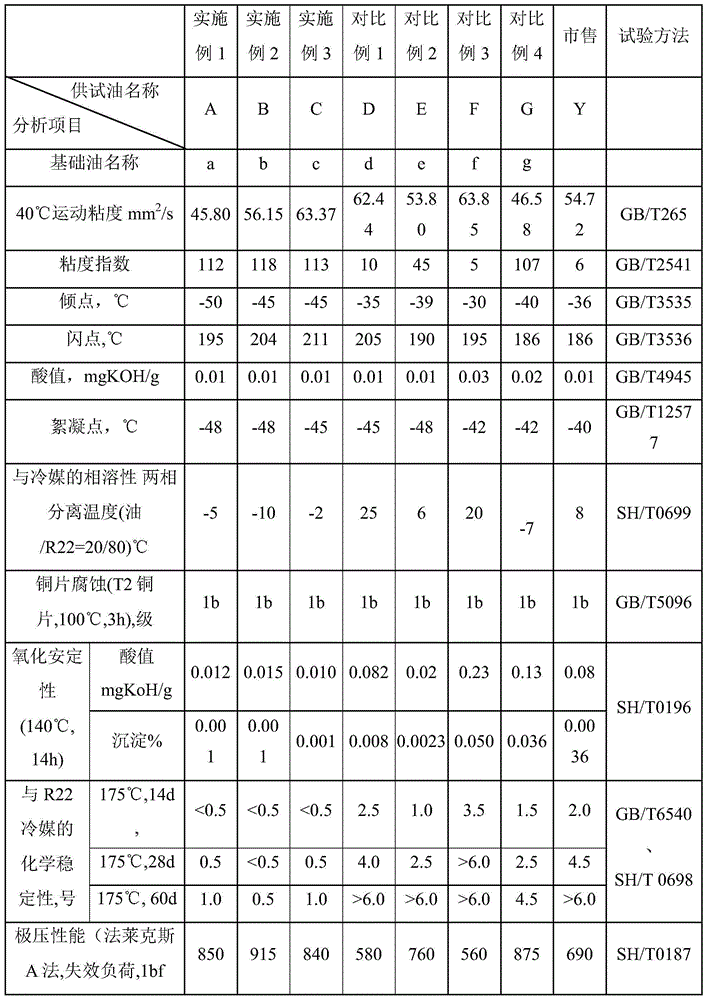
Refrigerant oil composition and application thereof
ActiveCN106147946AGood viscosity and temperatureIncreased viscosity-temperatureChemical industryHeat-exchange elementsChlorofluorocarbonHigh pressure
The invention provides a refrigerant oil composition and application thereof. The refrigerant oil composition comprises, on the basis of the weight of base oil, (1) 15 to 40 wt% of alkyl benzene synthetic lubricant base oil, (2) 15 to 40 wt% of oil-soluble polyether base oil, i.e., OSP base oil, (3) 20 to 70 wt% of high-pressure hydrogenation cycloalkyl mineral lubricant base oil, and (4) an additive, wherein the high-pressure hydrogenation cycloalkyl mineral lubricant base oil is prepared by using a high-pressure tandem all-hydrogen process consisting of hydrodemetallization, hydrotreatment, hydrodewaxing and hydrofinishing. The refrigerant oil provided by the invention has good intersolubility with hydrochlorofluorocarbons (HCFCs, such as R22), a low flocculation point and thermo-chemical stability; the refrigerant oil is especially applicable as refrigerant oil with HCFCs as refrigerants and also applicable as refrigerant oil with chlorofluorocarbons as refrigerants; and the refrigerant oil can prolong an oil change period and improve energy conservation performance, and has excellent abrasion resistance.
Owner:PETROCHINA CO LTD
New crystal form composition of cefminox sodium and preparation method thereof
ActiveCN102942576AHigh entropyImprove solubilityAntibacterial agentsOrganic active ingredientsCEFMINOX SODIUMBioavailability
The invention provides a new crystal form composition of cefminox sodium and a preparation method thereof, relating to the technical field of medicines and preparation method of medicines. The new crystal form composition of cefminox sodium is measured by powder X-ray diffraction, and the powder X-ray diffraction spectrum represented by a diffraction angle of 2 theta + / - 0.2 degrees has no specific diffractive peaks. The new crystal form is amorphous, and is fused at 171-173 DEG C. According to the invention, the medicine of the crystal form has the advantages of high entropy, good dissolvability, high bioavailability, and good dissolving stability; by using novel freeze drying technology, the operation is simple, the method is suitable for industrial production; and the disadvantages of slow dissolving speed, bad stability, and solid precipitates after long time storage of cefminox sodium crystals prepared by the prior art can be solved.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
Glufosinate ammonium water-soluble granule
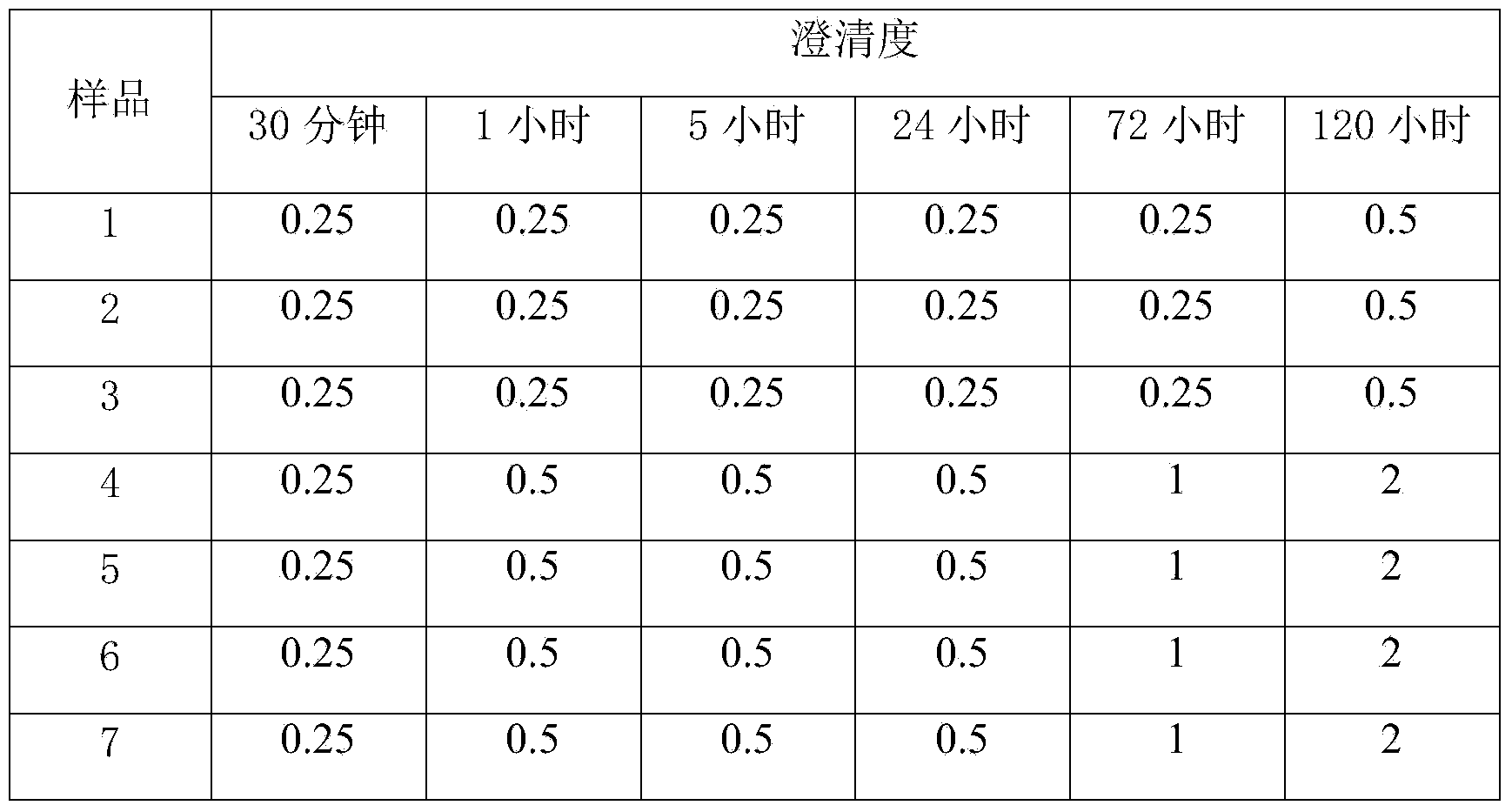
The present invention provides a glufosinate ammonium water-soluble granule, which comprises glufosinate ammonium, a surfactant and a carrier, wherein the glufosinate ammonium water-soluble granule further comprises a chelating agent and a synergist. According to the present invention, the type of the auxiliary agent of the glufosinate ammonium water-soluble granule is regulated, and the proper auxiliary material is combined and supplemented, such that various components synergistically act so as to obtain the glufosinate ammonium pesticide capable of achieving excellent water solubility, excellent dissolution stability, low foaming property and efficacy in high-concentration hard water, wherein the obtained glufosinate ammonium pesticide is especially suitable for countries and regions with high water hardness.
Owner:SHANDONG WEIFANG RAINBOW CHEM
Doxofylline crystalline compound and lyophilized powder thereof
InactiveCN103145713AImprove solubilityNot easy to precipitatePowder deliveryOrganic chemistryDoxofyllineX-ray
The invention relates to a doxofylline crystalline compound and lyophilized powder thereof. The doxofylline crystalline compound is measured by a powder X-ray diffraction measurement method; and an X-ray powder diffraction pattern represented by a diffraction angle of 2(theta)+ / -0.2 degrees shows characteristic diffraction peaks at the positions of 10.21 degrees, 12.12 degrees, 14.39 degrees, 23.32 degrees, 24.43 degrees, 27.23 degrees, 28.60 degrees, 30.54 degrees, 32.28 degrees, 33.32 degrees, 34.38 degrees and 36.12 degrees. The re-dissolubility of the doxofylline crystalline compound provided by the invention is obviously improved, solid is not easily separated out after long-time placement, and the medication safety of a patient is greatly improved.
Owner:SHANXI PUDE PHARMA CO LTD
Process of making stable abuse-deterrent oral formulations
ActiveUS20170368057A1Good dissolution stabilityOrganic active ingredientsCapsule deliveryAbuse deterrentDrug
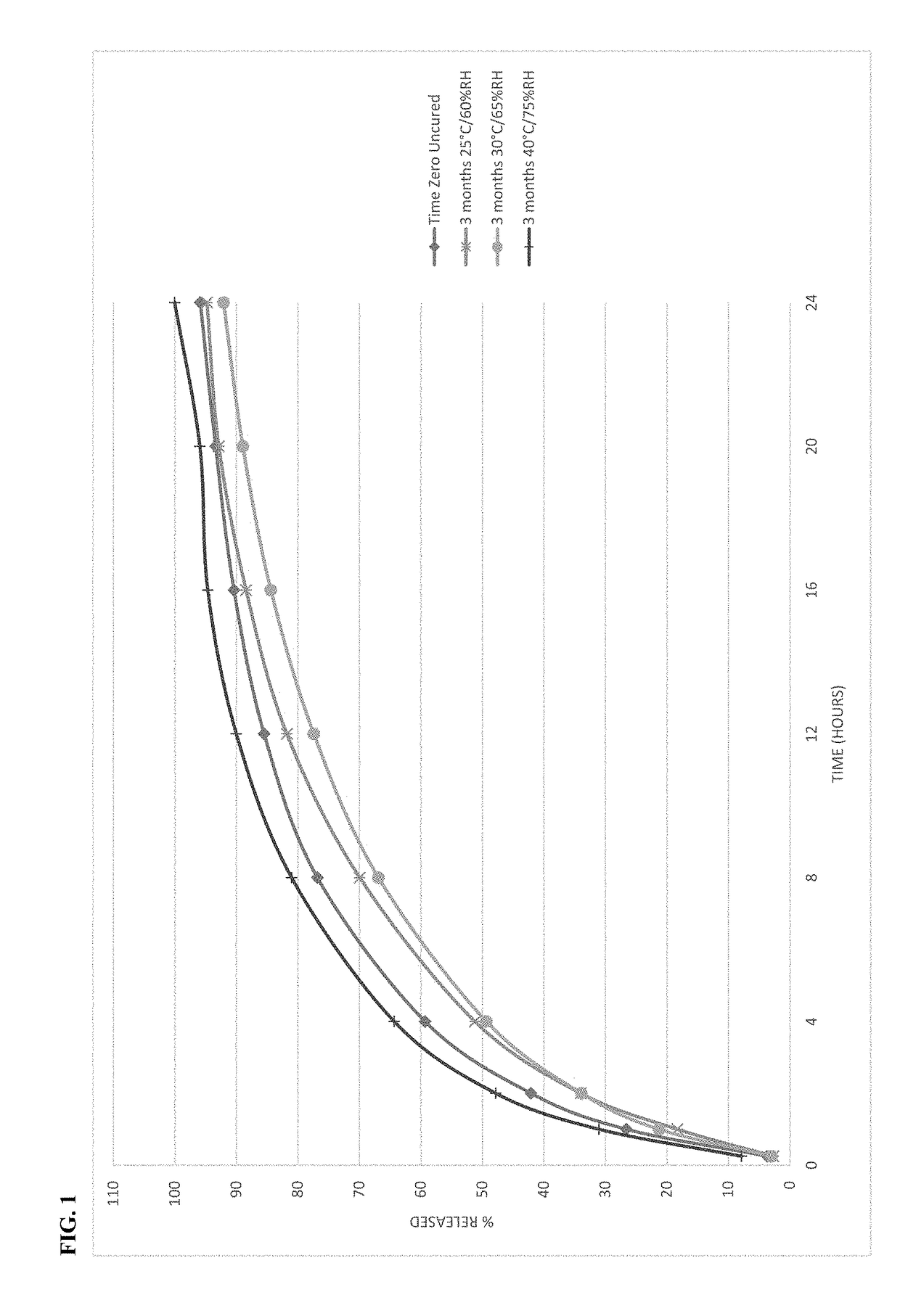
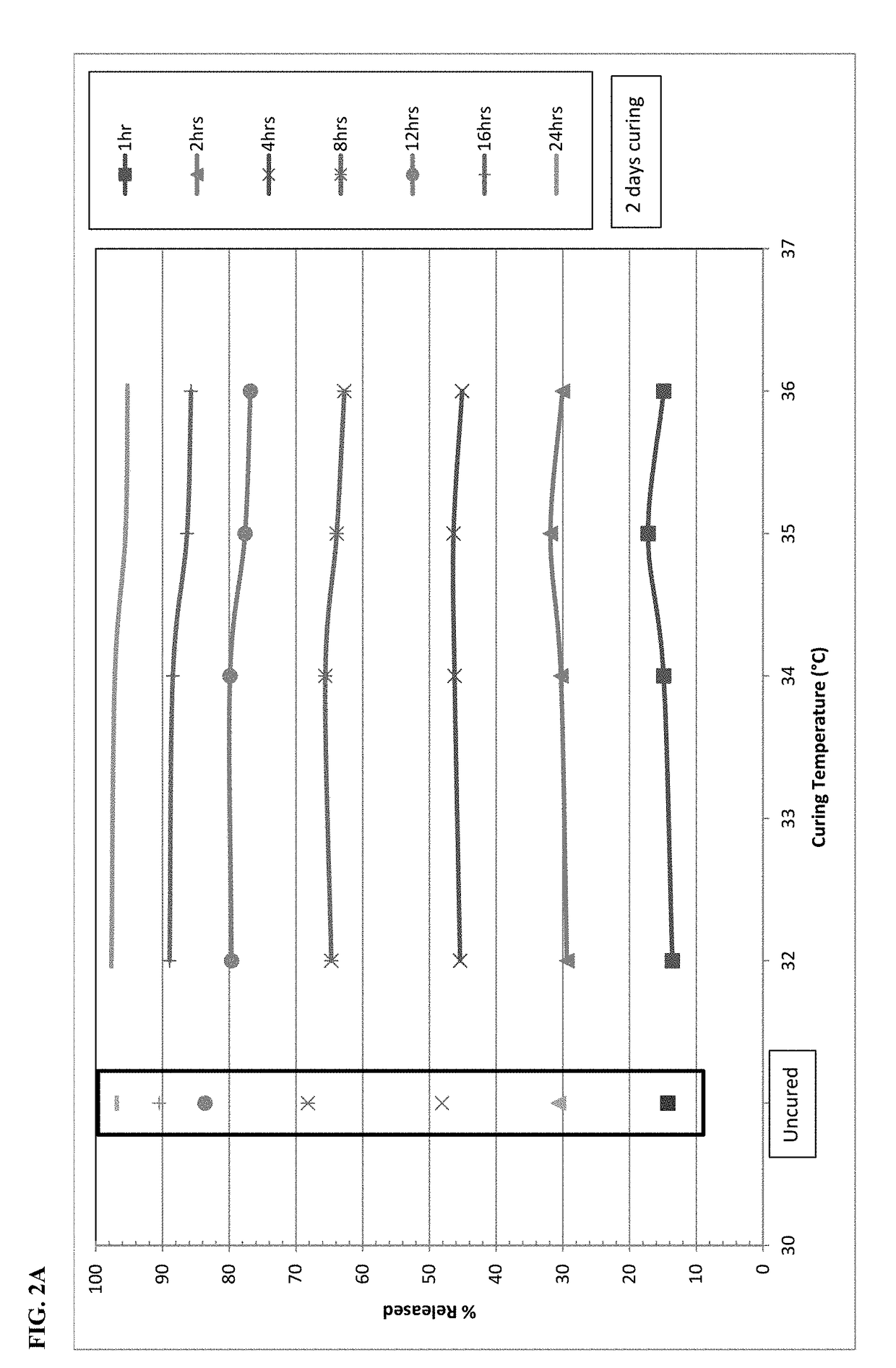
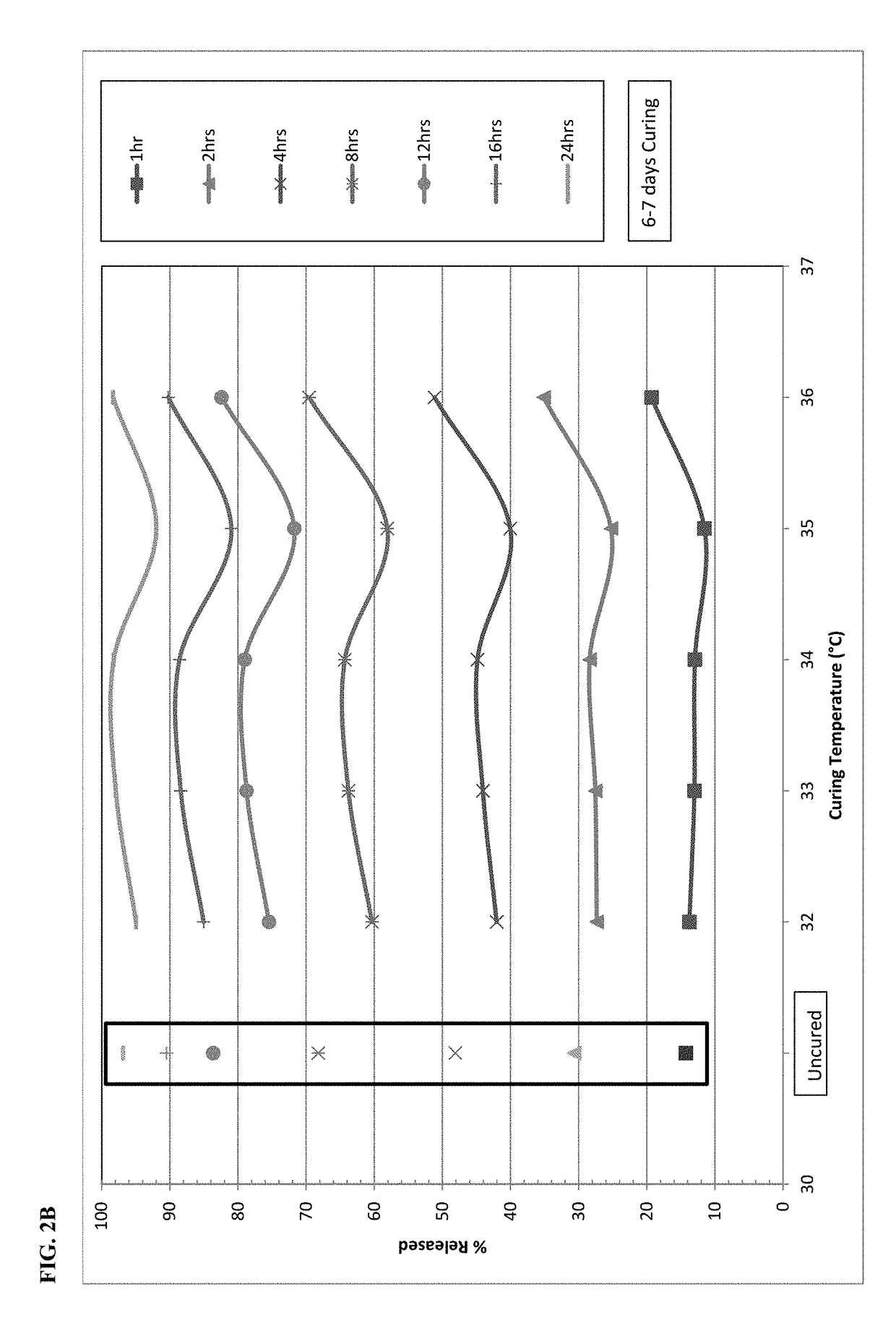
The present disclosure relates to cured pharmaceutical compositions designed to reduce the potential for improper administration of drugs that are subject to abuse, the process of curing such composition in order to improve the dissolution stability, method of using the same for treatment of pain.
Owner:COLLEGIUM PHARMA INC
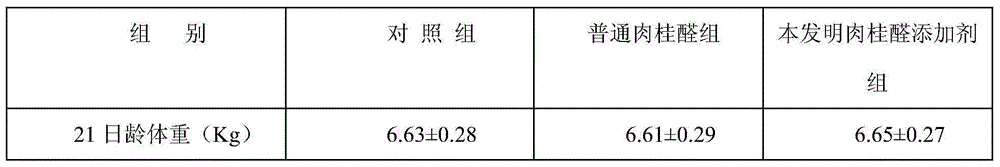
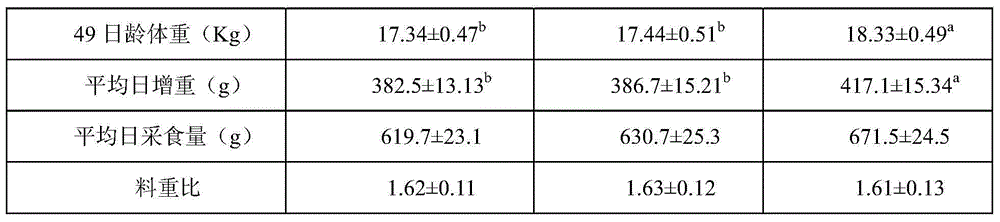
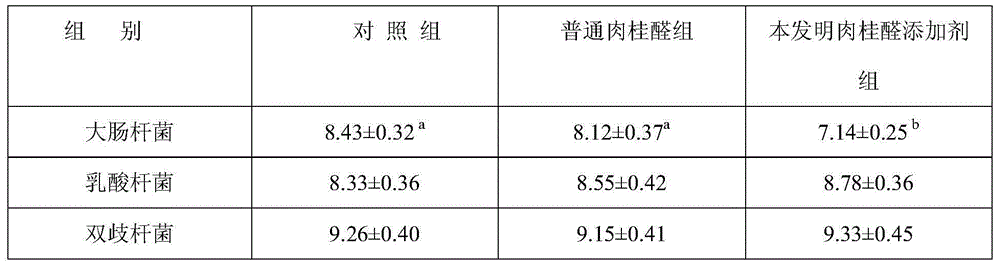
Preparation method of water-soluble cinnamic aldehyde additive for livestock and poultry
The invention discloses a preparation method of a water-soluble cinnamic aldehyde additive for livestock and poultry. The preparation method comprises the following steps: (1) mixing and homogenizing cinnamic aldehyde and an emulsifying agent so as to obtain a cinnamic aldehyde emulsion; (2) taking polyethylene glycol, and heating the taken polyethylene glycol so that the polyethylene glycol is melted; (3) adding the cinnamic aldehyde emulsion to the melted polyethylene glycol, and shearing and stirring the cinnamic aldehyde emulsion and the melted polyethylene glycol for 10-20 minutes at a high speed; (4) leaving the mixture of the cinnamic aldehyde emulsion and the melted polyethylene glycol to stand for 10-15 minutes, performing filtration, and collecting filtrate; (5) sending the filtrate into a pressure type spray drying tower, and condensing, spraying and pelleting the filtrate so as to obtain granular solids; and (6) classifying the obtained granular solids with a vibrating screen, and screening the classified granular solids through a sieve of 20-60 meshes so as to obtain the water-soluble cinnamic aldehyde additive. According to the preparation method disclosed by the invention, the volatilization of a cinnamic aldehyde preparation in the using process is reduced, the prepared cinnamic aldehyde additive is easy to dissolve in water and can be mixed and dissolved with chyme in intestinal tracts of animals, the bacterial inhibition of the cinnamic aldehyde additive in the intestinal tracts of the animals can be improved, the stability of the cinnamic aldehyde additive after the cinnamic aldehyde additive is dissolved in the water is good, and the cinnamic aldehyde additive can be used as a preparation for drinking water, so that the application range of the products is extended.
Owner:ZHEJIANG WANFANG BIO TECH CO LTD
Preparation method of azithromycin freeze-drying agent for injection
PendingCN111803455AImprove stabilityImprove solubilityAntibacterial agentsOrganic active ingredientsActivated carbonSolvent
The invention provides a preparation method of an azithromycin freeze-drying agent for injection. The method comprises the following steps of S1, mixing and dissolving raw materials: adding a cosolvent into water for injection, adding a pH regulator to regulate the pH value to 5.0-5.2, adding azithromycin into the water for injection, and performing stirring until the azithromycin is dissolved toform a mixed liquid medicine A; S2, performing decolorizing and impurity removing: regulating the pH value to 6.0-7.0 by adopting the pH regulator, adding activated carbon for needles, performing stirring for 15-20 min at the room temperature, and performing sterilizing, filtering and decarbonizing to form a mixed liquid medicine B; and S3, performing freeze-drying: a, repeatedly performing pre-freezing, b, performing primary sublimation drying, and c, performing drying again, wherein the cosolvent is citric acid, sodium hydroxide, mannitol and cis-6-nonen-1-ol, and the raw materials include the following components in parts by weight: 100 parts of the azithromycin, 50-60 parts of the citric acid, 20-30 parts of the sodium hydroxide, 30-40 parts of the mannitol, 3-5 parts of the cis-6-nonen-1-ol and 1500-2000 parts of the water for injection. The preparation method of the azithromycin freeze-drying agent for injection is good in solubility and high in stability, and the clarity of a prepared injection solution is high.
Owner:湖北潜龙药业有限公司
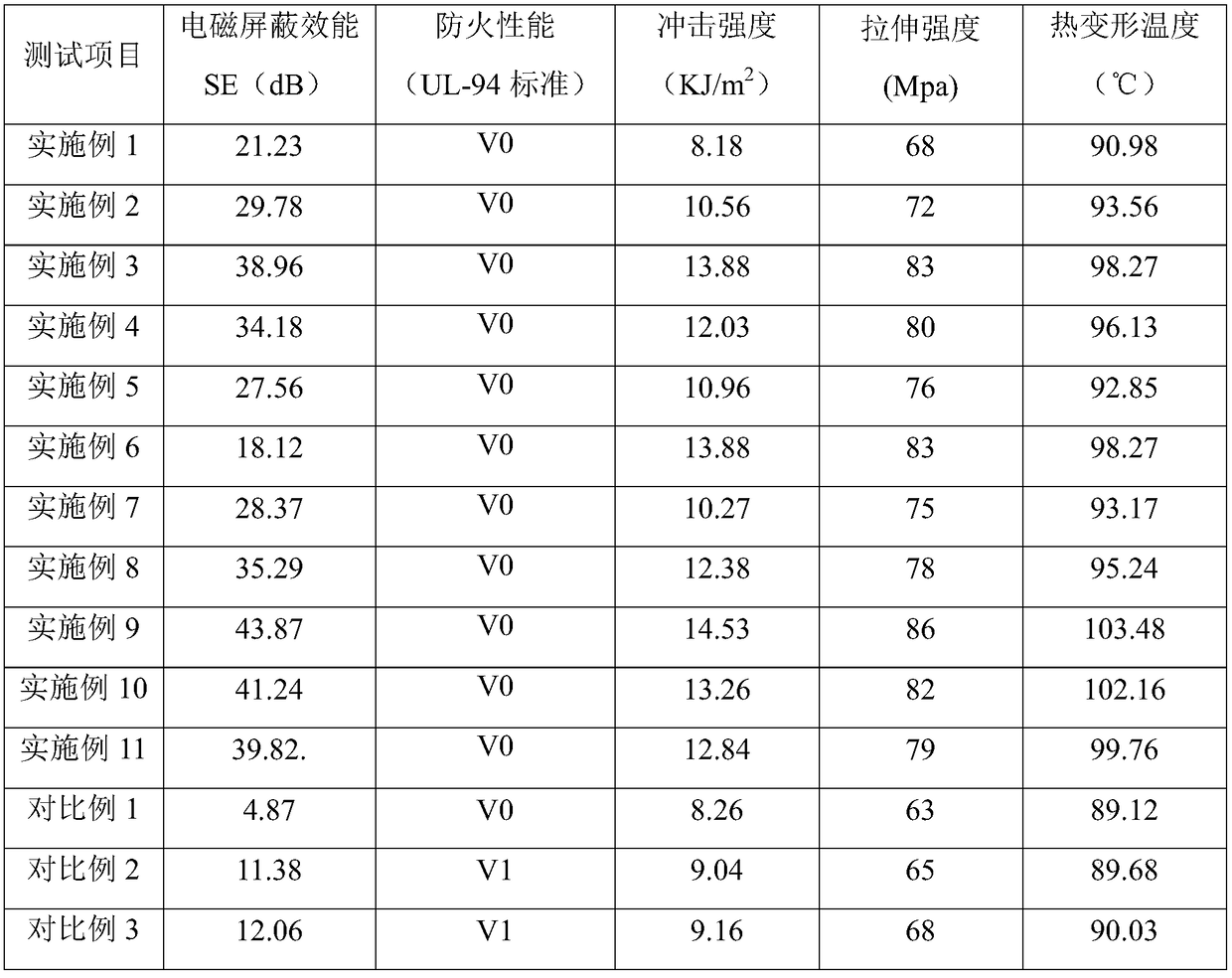
Electromagnetic shielding material, preparation method thereof and cable protection tube produced by electromagnetic shielding material
InactiveCN109021421AGood electromagnetic shielding effectStrong impact resistanceFiberOxide composite
The invention relates to the technical field of cable materials, in particular to an electromagnetic shielding material, a preparation method thereof and a cable protection tube produced by the electromagnetic shielding material. The electromagnetic shielding material comprises the following raw materials in parts by weight: 75-100 parts of PVC resin, 12-18 parts of an electromagnetic shielding material, 15-20 parts of a flame retardant, 8-12 parts of a synergistic flame retardant, 10-15 parts of an impact resistance agent, 12-18 parts of a coupling agent, 5-15 parts of a compatibilizer, and 6-10 parts of acrylate fiber, wherein the electromagnetic shielding material is a polyaniline-graphene oxide composite material. The PVC material of the invention has better properties of flame retardancy, impact resistance, weather resistance, tensile strength and hardness. The electromagnetic wave is reflected by utilizing the high conductivity properties of polyaniline, the high absorbing properties of graphene oxide is combined to absorb electromagnetic waves, so that the PVC material has excellent electromagnetic shielding effect.
Owner:SHENZHEN ANPOWER ELECTRIC
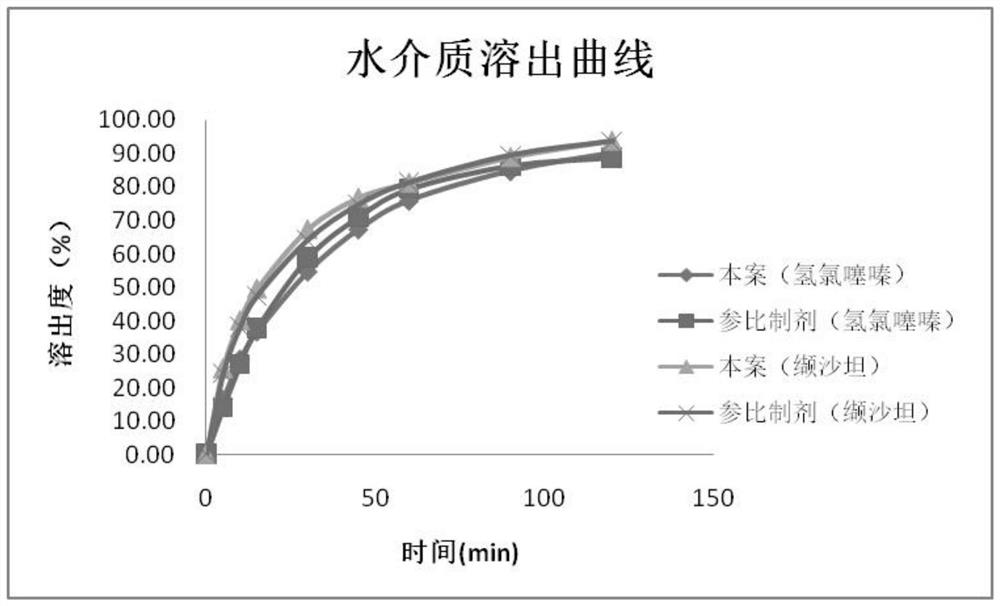
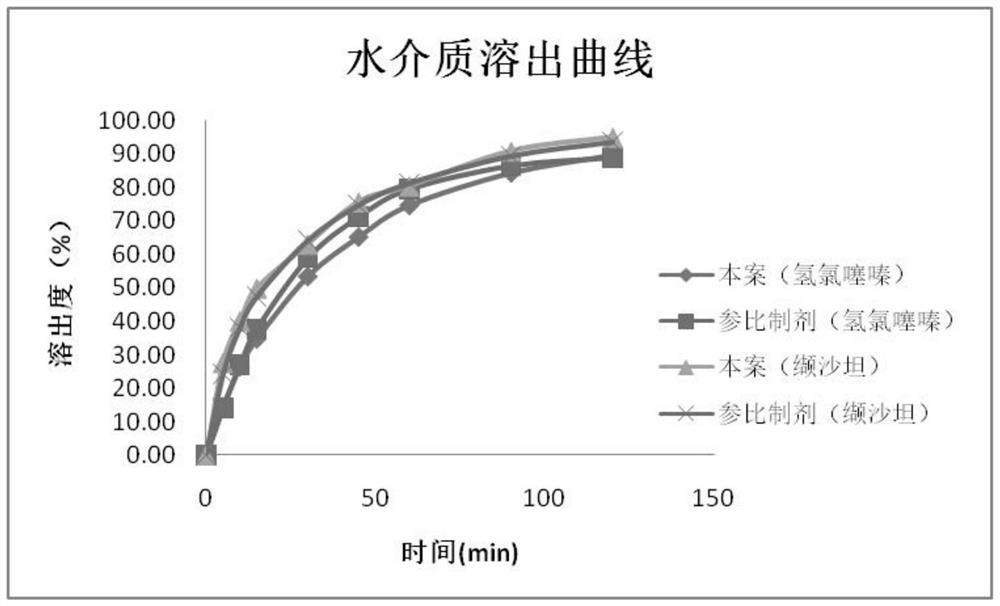
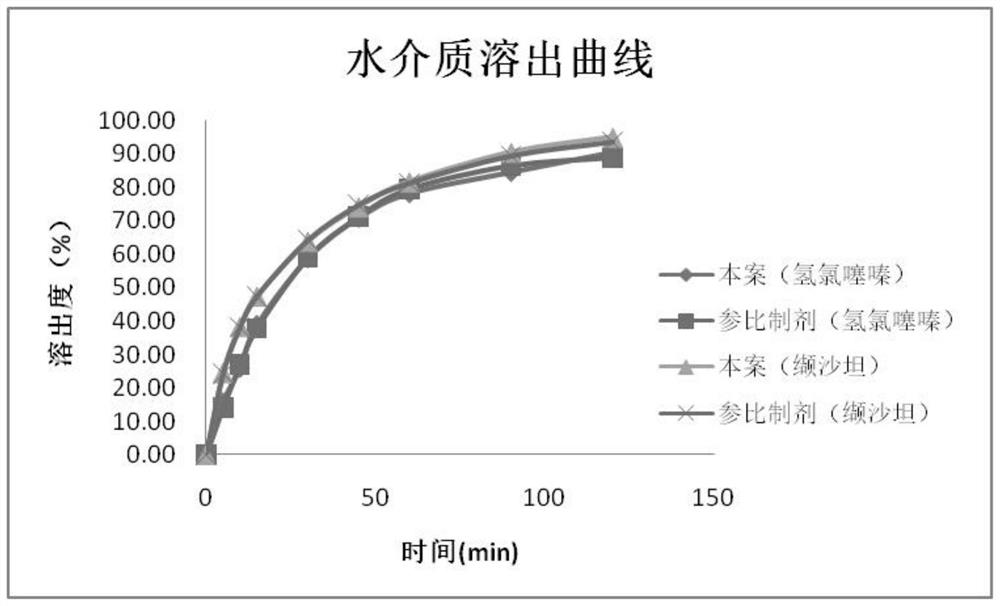
Valsartan and hydrochlorothiazide compound preparation and preparation process thereof
ActiveCN113041250AImprove solubilityImprove bioavailabilityPharmaceutical product form changePharmaceutical non-active ingredientsValsartanAdhesive
The invention discloses a valsartan and hydrochlorothiazide compound preparation and a preparation process thereof. The preparation process comprises the following steps: pretreating raw materials; pretreating auxiliary materials; preparing dispersion: dispersing valsartan on the surface of the carrier by adopting a supercritical fluid impregnation technology; preparing inclusion compound: carrying out inclusion on hydrochlorothiazide by adopting sulfobutyl ether-beta-cyclodextrin; preparing pre-coated particles: conducting top spraying granulation on the hydrochlorothiazide inclusion compound through a composite adhesive; premixing: mixing the valsartan solid dispersion, the auxiliary materials and the hydrochlorothiazide pre-coated particles; distributing materials: dividing the premixed powder into two parts; respectively carrying out dry granulation on the two parts of premixed powder; mixing totally; tabletting; and coating to obtain the product. The compound preparation with good bioavailability and drug stability is prepared by combining a supercritical impregnation technology, a secondary inclusion technology and a different oil pressure powder granulation technology, the production cost is low, and the process is simple and easy to implement.
Owner:上海耀大生物科技有限公司
Cefoperazone sodium compound and medicine composition thereof
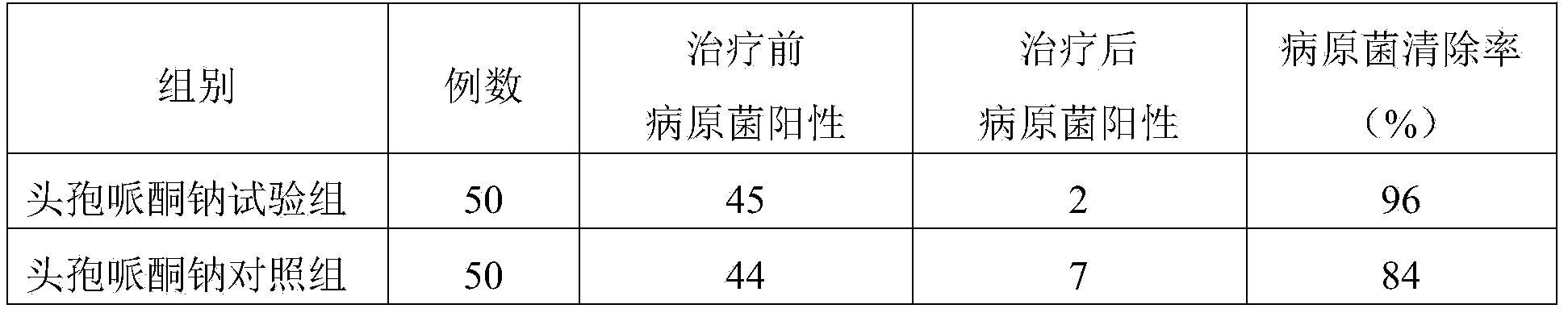
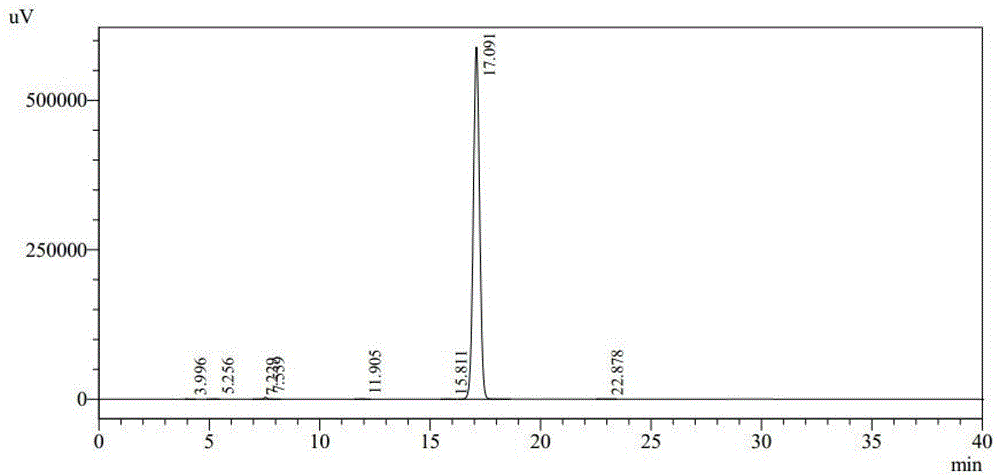
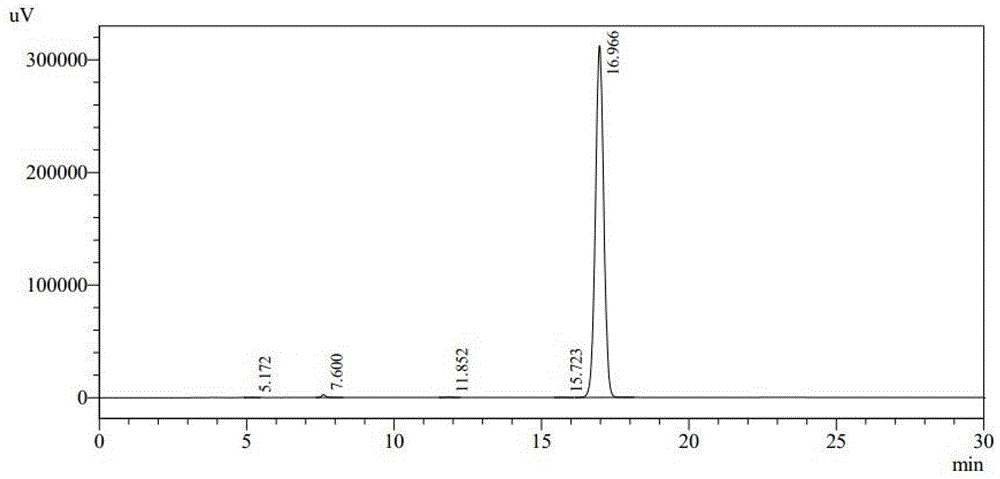
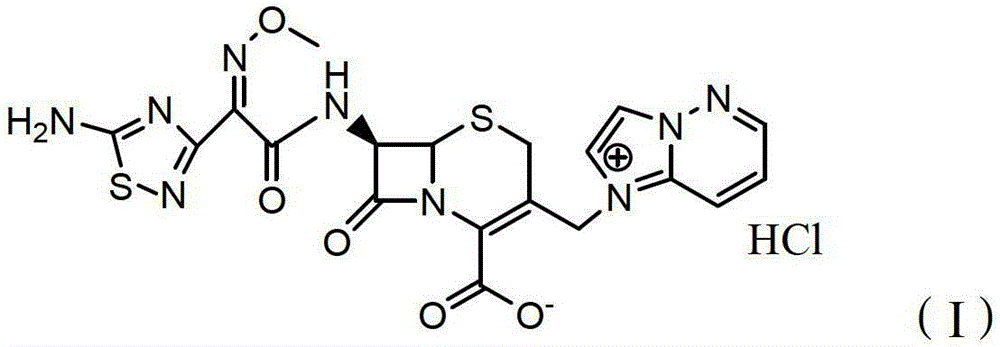
ActiveCN103951679AGood dissolution stabilityGood curative effectAntibacterial agentsOrganic active ingredientsSolubilityX-ray
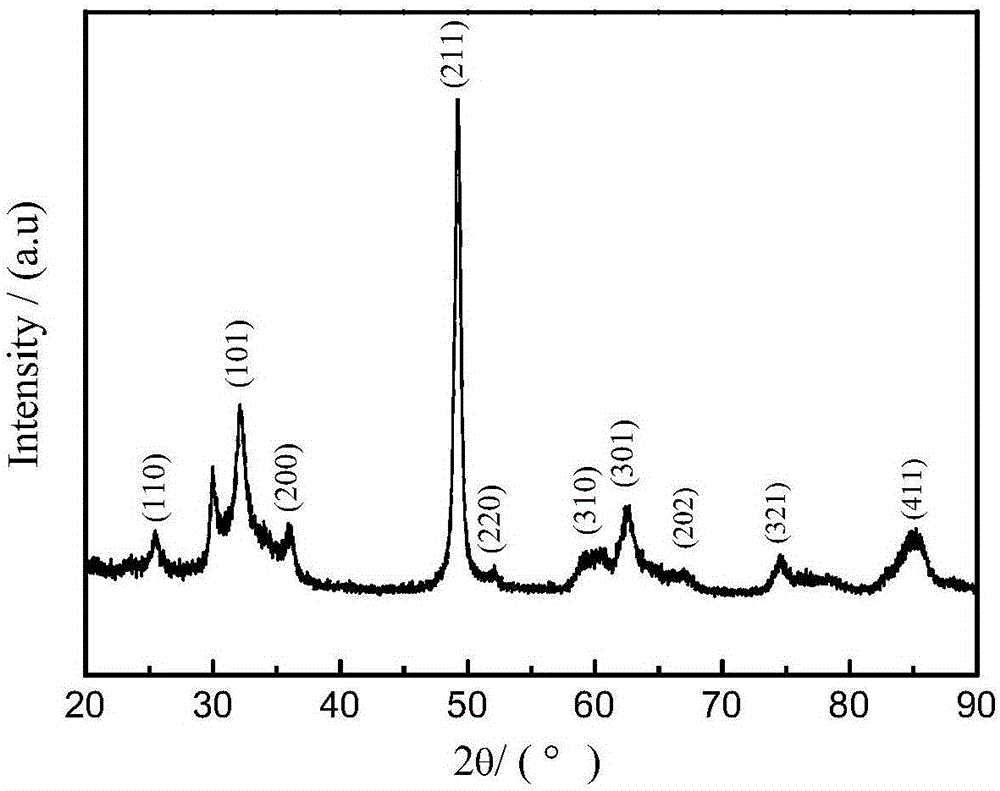
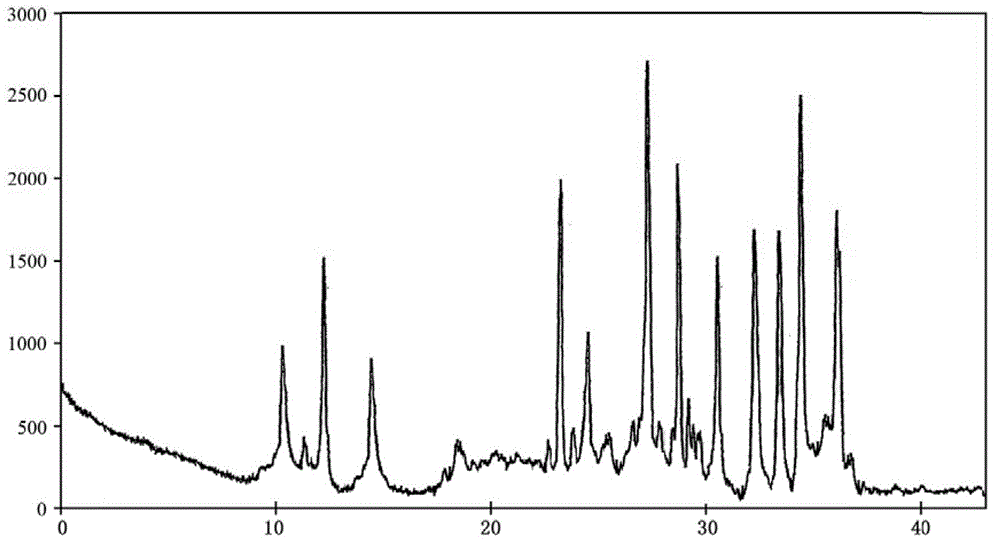
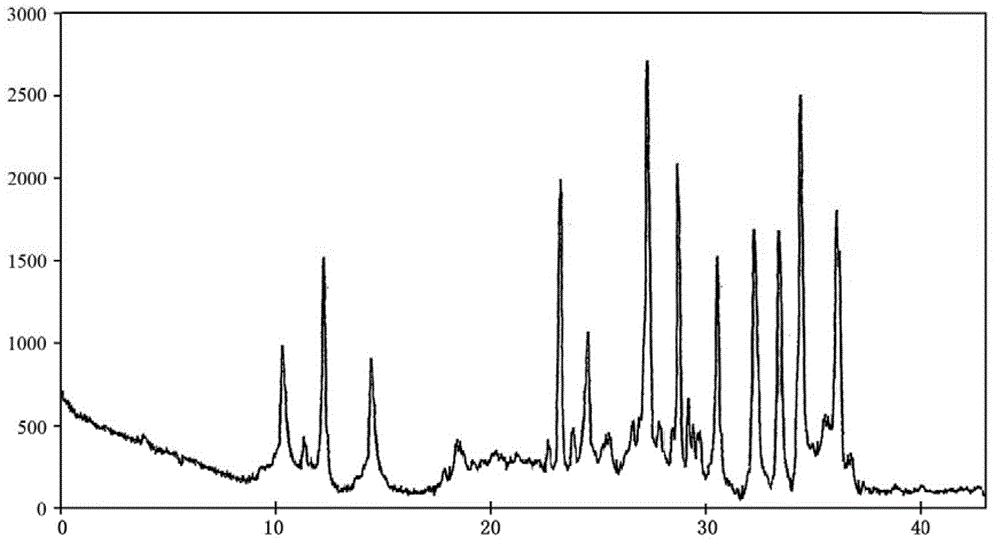
The invention belongs to the technical field of medicines, and particularly relates to a cefoperazone sodium compound and a medicine composition thereof. The structure formula of the cefoperazone sodium compound is as shown in a formula (I). The compound is determined by using a powder X-ray diffraction determination method, and an X-ray powder diffraction spectrum shown by a diffraction angle of 2 theta + / - 0.2 degree is as shown in a figure 1. The cefoperazone sodium compound provided by the invention has good solubility and stability, and solids can not be separated out after the cefoperazone sodium compound is placed for a long time; and in addition, the cefoperazone sodium compound has a better curative effect and a better clearing effect on pathogenic bacteria.
Owner:YOUCARE PHARMA GROUP +1
Method for producing goat milk desalinated whey powder
The invention relates to a method for producing goat milk desalinated whey powder. The method comprises the following steps: purifying fresh goat milk, sterilizing the fresh goat milk, adding a citric acid solution and continuously stirring the materials, then centrifuging the materials, removing fat in the goat milk to obtain whey liquid, using food-grade calcium hydrate for adjusting the pH value of the whey liquid to 6.5-6.8, employing a desalination combination machine to removing the partial salinity in the whey liquid, concentrating the material, and performing spray drying to obtain the goat milk desalinated whey powder. The method greatly increases the dissolving stability of the goat whey powder products, the produced desalinated goat whey powder can reach the national standard of whey powder, and can satisfy the requirement of the desalinated goat whey powder by goat milk enterprises.
Owner:建水县鸿辉种养殖产业有限公司
Polishing composition and polishing method using the polishing composition
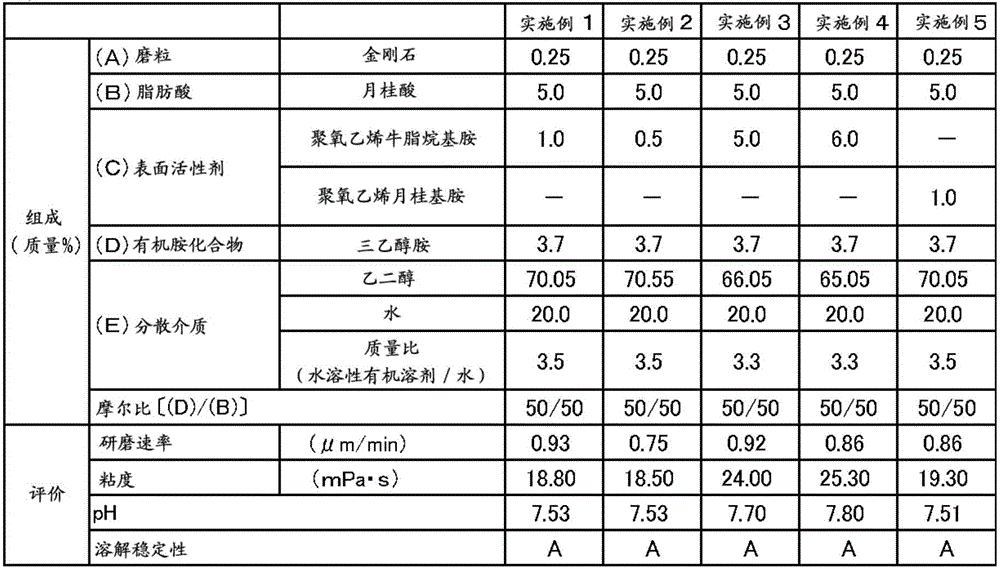
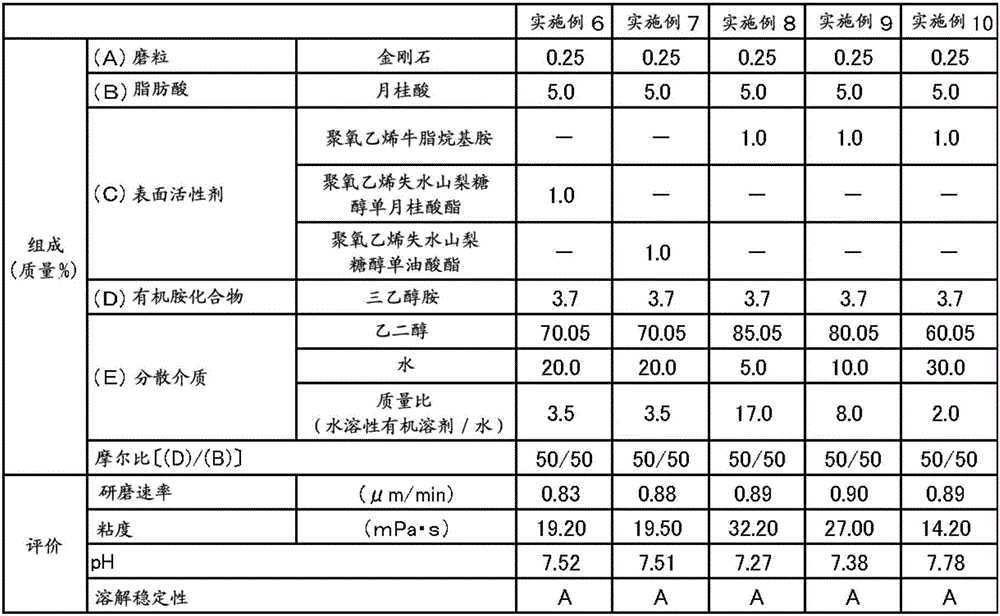
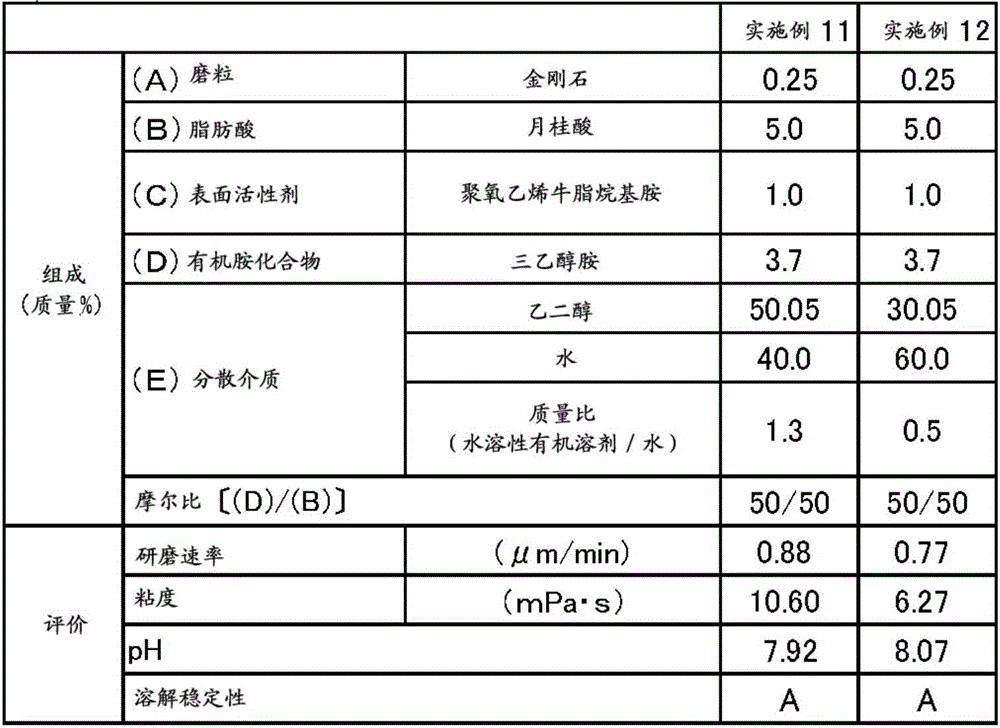
ActiveCN106167691AIncrease grinding rateGood dissolution stabilityOther chemical processesSemiconductor/solid-state device manufacturingBoron carbideBoron nitride
The present invention provides a polishing composition which is excellent in dissolution stability and capable of polishing at a high polishing rate, and a polishing method using the polishing composition. Thus, there is provided a method of polishing a substrate comprising at least one material selected from the group consisting of sapphire, silicon carbide, gallium nitride and aluminum nitride using a polishing composition containing the following components (A) to (E) (A): one or more abrasive grains selected from the group consisting of diamond, boron nitride, boron carbide and silicon carbide, (B) a fatty acid having more than 10 and less than 22 carbon atoms, and (C) Nonionic surfactant, (D) component: organic amine compound, (E) dispersion medium. The abrasive grains of the component (A) have an average particle size which is greater than 1.0 [mu]m and less than 10.0 [mu]m, the content of the component (C) is 0.30 to 10% by mass, and the the molar ratio (D) / (B) between (D) and (B) is from 45 / 55 to 90 / 10.
Owner:SHOWA DENKO KK
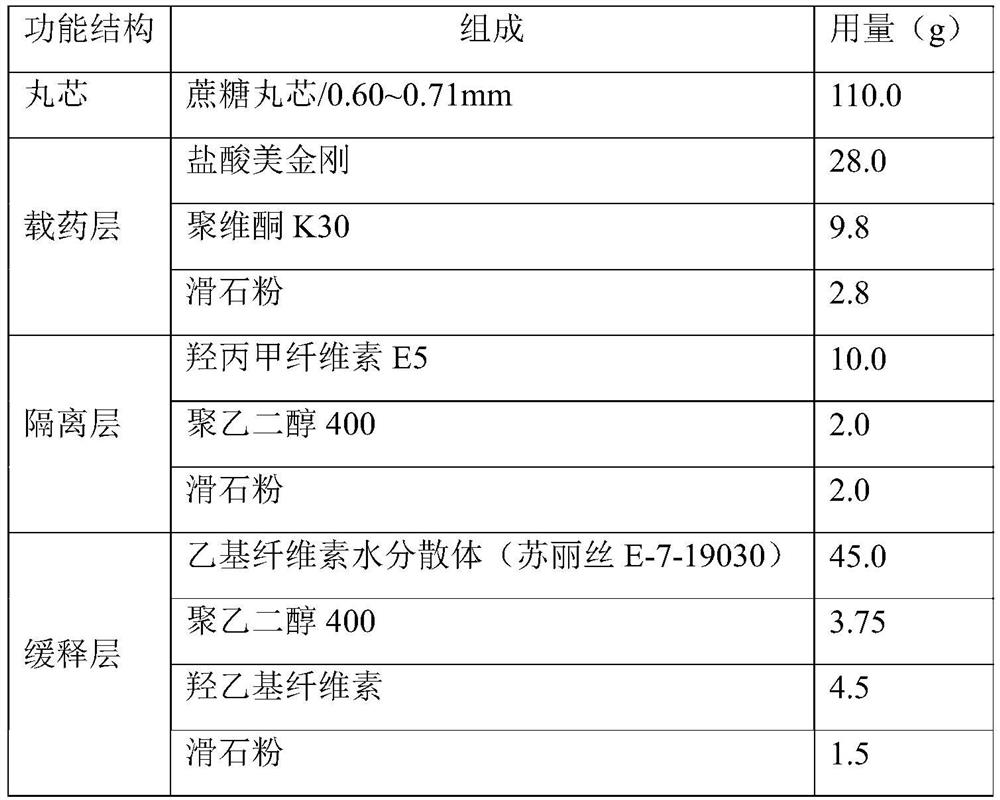
Memantine hydrochloride sustained-release pellet and preparation method thereof
ActiveCN114748443AGood dissolution stabilityGood hygroscopicityNervous disorderPill deliverySustained release pelletsMemantine Hydrochloride
The invention belongs to the field of pharmaceutical preparations, and particularly relates to a memantine hydrochloride-containing sustained-release pellet and a preparation method thereof. The memantine hydrochloride sustained-release pellet comprises a blank pellet core, a drug-loading coating layer, an isolation coating layer and a sustained-release coating layer, and a sustained-release material of the sustained-release coating layer is mainly composed of an ethyl cellulose aqueous dispersion and contains a proper proportion of hydroxyethyl cellulose. The memantine hydrochloride sustained-release pellet can be further prepared into sustained-release preparations such as granules, capsules or tablets. According to the memantine hydrochloride sustained-release pellet and the preparation method thereof, further aggregation and fusion of ethyl cellulose are retarded by utilizing an incompatible membrane formed by dispersedly wrapping hydrophobic ethyl cellulose with water-soluble hydroxyethyl cellulose and water absorption of the incompatible membrane, so that the memantine hydrochloride sustained-release pellet with better stability is obtained, no organic solvent such as ethanol is used, and the memantine hydrochloride sustained-release pellet is more economical, environment-friendly and safe and has good application prospects. The method is suitable for industrial production.
Owner:北京丰科睿泰医药科技有限公司
Anti-freezing early-strength alkali-free liquid setting accelerator and preparation method thereof
ActiveCN113045236AGood dissolution stabilityEliminate the need for insulationInorganic saltsFluid phase
The invention discloses an anti-freezing early-strength alkali-free liquid setting accelerator and a preparation method thereof. The setting accelerator is prepared by mixing a setting adjusting component, a solubilizing component and an anti-freezing early strength component; the setting adjusting component comprises aluminum sulfate, fluosilicate, alkylol amine, a stabilizer and water, the solubilizing component comprises amino acid and water, and the anti-freezing early strength component comprises nitrate, sulfonic acid and water. According to the setting accelerator disclosed by the invention, the freezing point of an accelerator solvent water is reduced, aluminum ions are inhibited from being hydrolyzed into aluminum hydroxide gel precipitates, and the dissolving stability of inorganic salts such as aluminum sulfate is improved, so that the accelerator does not freeze and lose efficacy in a low-temperature environment, and the heat preservation requirement in the transportation and storage process is avoided; nitrate is adopted to improve the formation of cement hydration liquid phase ions and promote the formation of hydration products; meanwhile, the sulfonic acid can reduce the retarding effect of ettringite on inhibiting cement hydration and promote hydration of cement minerals; and through the synergistic effect of the two components, the setting and hardening process of the cement is accelerated, and the quick-hardening and early-strength effects at low temperature are realized.
Owner:JIANGSU SOBUTE NEW MATERIALS +2
High-content ceramide repairing cream and preparation method thereof
ActiveCN111568829AImprove stabilityStrengthens the skin barrierCosmetic preparationsToilet preparationsBiotechnologySkin barrier function
The invention discloses high-content ceramide repairing cream and a preparation method thereof, and relates to the technical field of cosmetics. The high-content ceramide repairing cream is prepared from the following components in percentage by mass: 5 to 30 percent of polyhydric alcohol, 0.2 to 15 percent of ceramide, 5-35% of an oil phase substance, 0.5 to 3.5 percent of lecithin, 1.0 to 12 percent of a functional composition and the balance of water. According to the high-content ceramide repairing cream provided by the invention, the content of ceramide is as high as 15%, and product stability is good. The high-content ceramide is matched with the functional composition, so that the skin barrier function can be synergistically enhanced, and the repairing effect is obvious. According to the preparation method of the high-content ceramide repairing cream provided by the invention, a special emulsification process is adopted, so that the dissolving stability of ceramide can be effectively improved, the adding amount of grease for dissolving ceramide can be greatly reduced, the adding amount of oil-phase substances is low, and the preparation of refreshing skin feeling cream is facilitated.
Owner:SHANGHAI NEW COGI COSMETIC
Preparation method of freeze-dried preparation of cefozopran hydrochloride
ActiveCN104095819ALess impuritiesQuality improvementAntibacterial agentsOrganic active ingredientsSodium carbonate anhydrousCarbon dioxide
The invention relates to a preparation method of an injection-use freeze-dried preparation of cefozopran hydrochloride. The method includes following steps: (1) dissolving anhydrous sodium carbonate and sodium chloride in water at 20-40 DEG C and adjusting a pH value to 8.0-10.0 with carbon dioxide to obtain an auxiliary material solution; (2) dissolving cefozopran hydrochloride, or a solvate thereof, in an ethanol-water solution, or a methanol-water solution, to obtain a cefozopran hydrochloride solution, wherein the ethanol-water solution, or the methanol-water solution, is pre-heated to 35-55 DEG C; (3) adding dropwisely the auxiliary material solution prepared in the step (1) to the cefozopran hydrochloride solution prepared in the step (2) with the pH value being controlled within 7.0-8.5 through carbon dioxide to obtain a mixed solution; (4) adding needle-use activated carbon to the mixed solution with stirring with carbon dioxide being added; and (5) filtering a solution obtained through the step (4) to obtain a sterile filtrate and performing a freeze-drying process. By means of the method in the invention, the freeze-dried preparation of cefozopran hydrochloride is little in impurities, is high in quality and can satisfy, even is higher than a standard in the 15th version of the Japanese pharmacopoeia.
Owner:珠海保税区丽珠合成制药有限公司
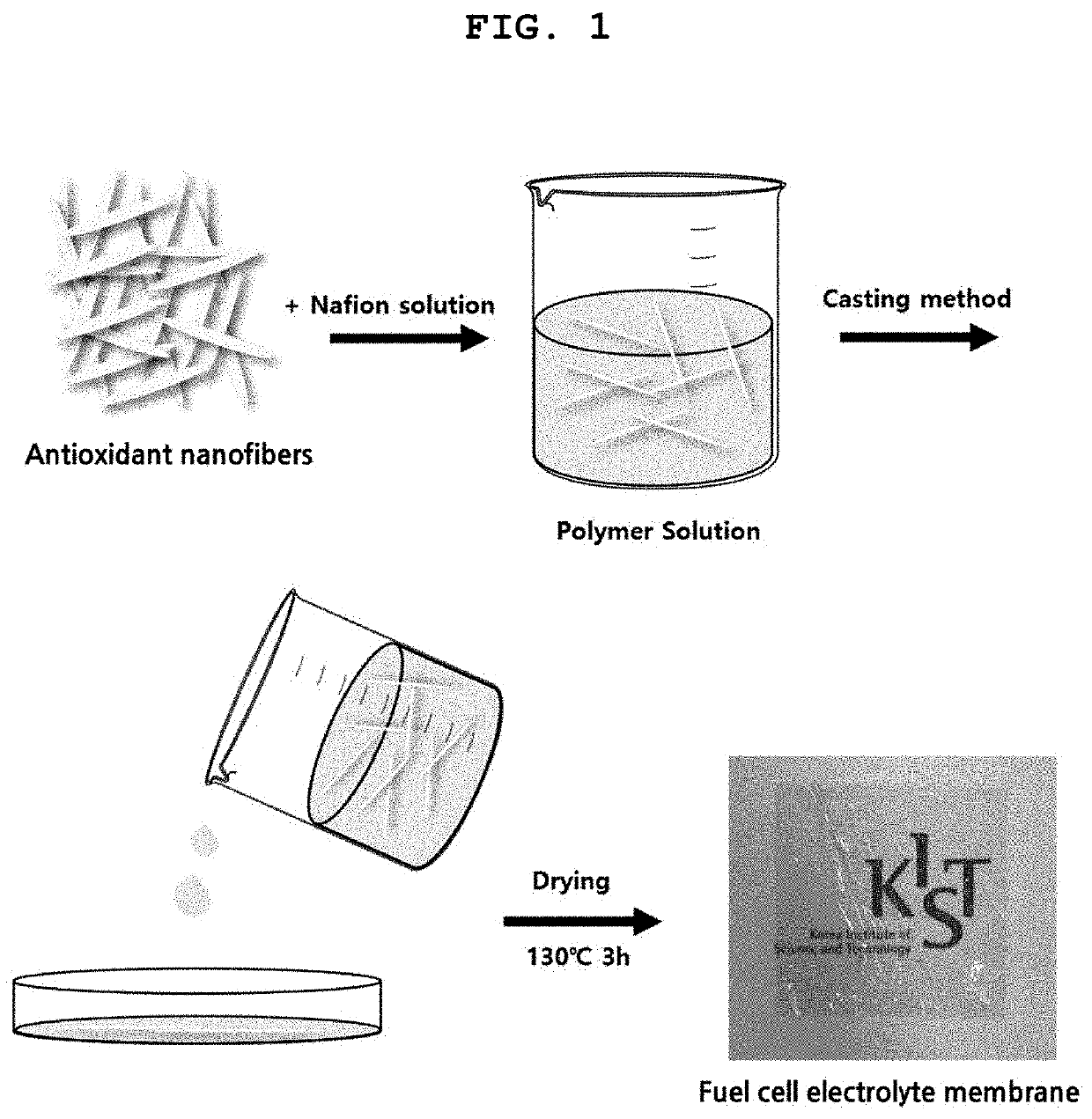
Antioxidant for electrolyte membrane of fuel cell and method for preparing the same
ActiveUS20220021015A1Improve conductivityHigh strengthSolid electrolytesFinal product manufacturePolymer electrolytesFuel cells
Disclosed is an antioxidant for a polymer electrolyte membrane of a fuel cell including cerium hydrogen phosphate (CeHPO4). The presence of cerium hydrogen phosphate in the antioxidant enhances the dissolution stability of cerium and improves the ability to capture water, leading to an increase in proton conductivity. In addition, the cerium hydrogen phosphate has a crystal structure composed of smaller cerium particles. This crystal structure greatly improves the ability of the antioxidant to prevent oxidation of the electrolyte membrane. Also disclosed are an electrolyte membrane including the antioxidant, a fuel cell including the electrolyte membrane, a method for preparing the antioxidant, a method for producing the electrolyte membrane, and a method for fabricating the fuel cell.
Owner:KOREA INST OF SCI & TECH
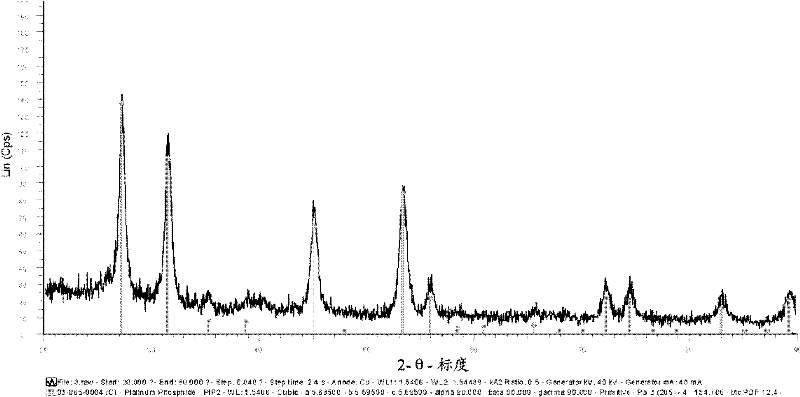
Platinum phosphide as a cathode catalyst for pemfcs and phosphorous treatment of catalysts for fuel cell
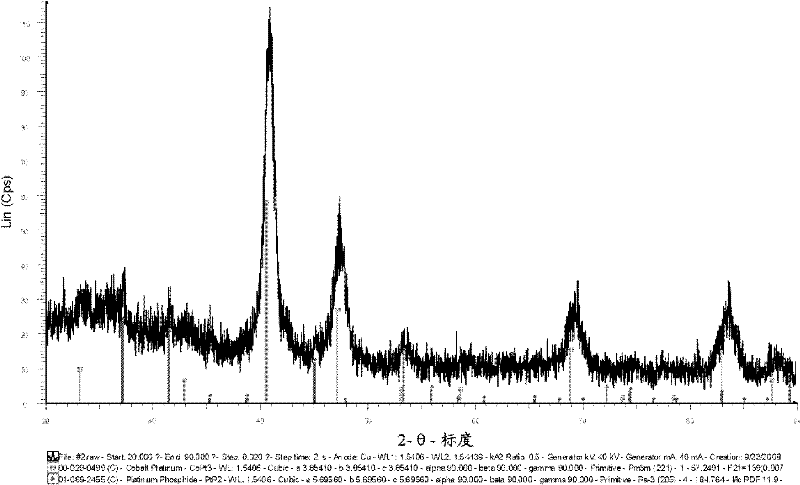
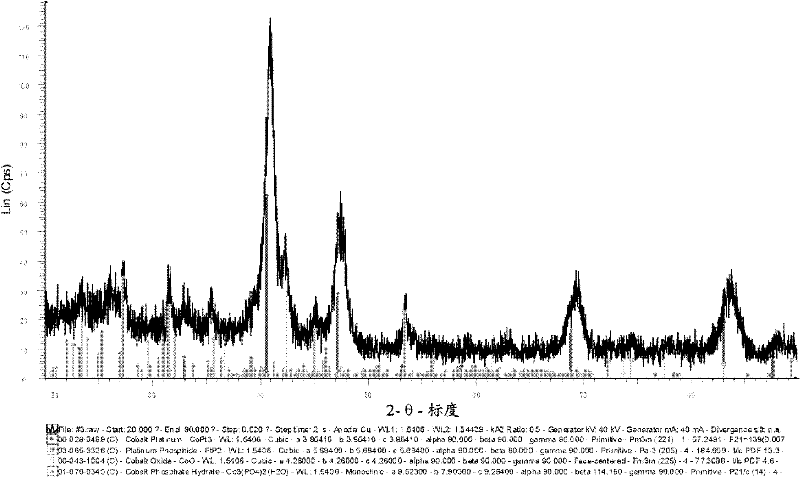
InactiveCN102365775AHigh activityReduce loadPhysical/chemical process catalystsCell electrodesPlatinum complexFuel cells
The present disclosure relates to a catalyst including platinum phosphide having a cubic structure, a method of making the catalyst, and a fuel cell utilizing the catalyst. The present disclosure also relates to method of making electrical power utilizing a PEMFC incorporating the catalyst. Also disclosed herein is a catalyst including a platinum complex wherein platinum is complexed with a nonmetal or metalloid. The catalyst with the platinum complex can exhibit good electrochemically active properties.
Owner:CELLCENTRIC GMBH & CO KG +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com