Valsartan and hydrochlorothiazide compound preparation and preparation process thereof
A preparation technology of hydrochlorothiazide, which is applied in the field of pharmaceutical preparations, can solve the problems of low bioavailability, increased degradation products, and low dissolution rate in vitro, and achieve the effects of improving dissolution rate and solubility, improving stability, and good miscibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
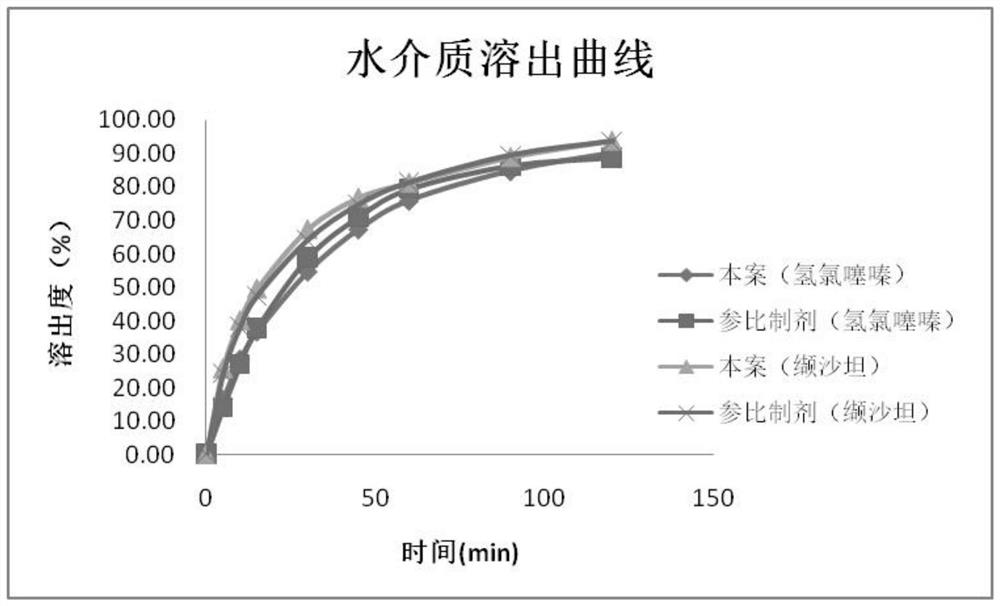
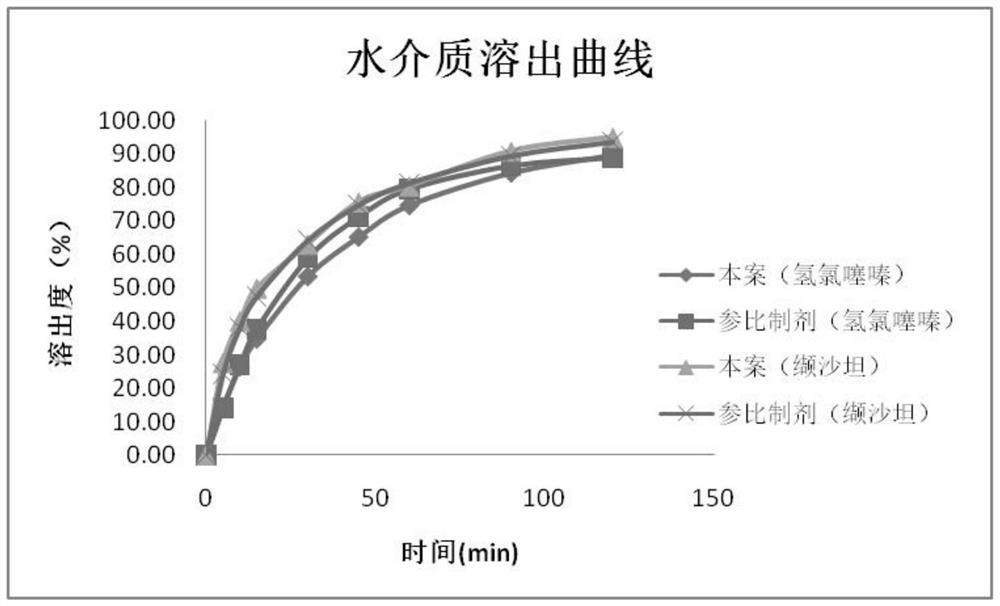
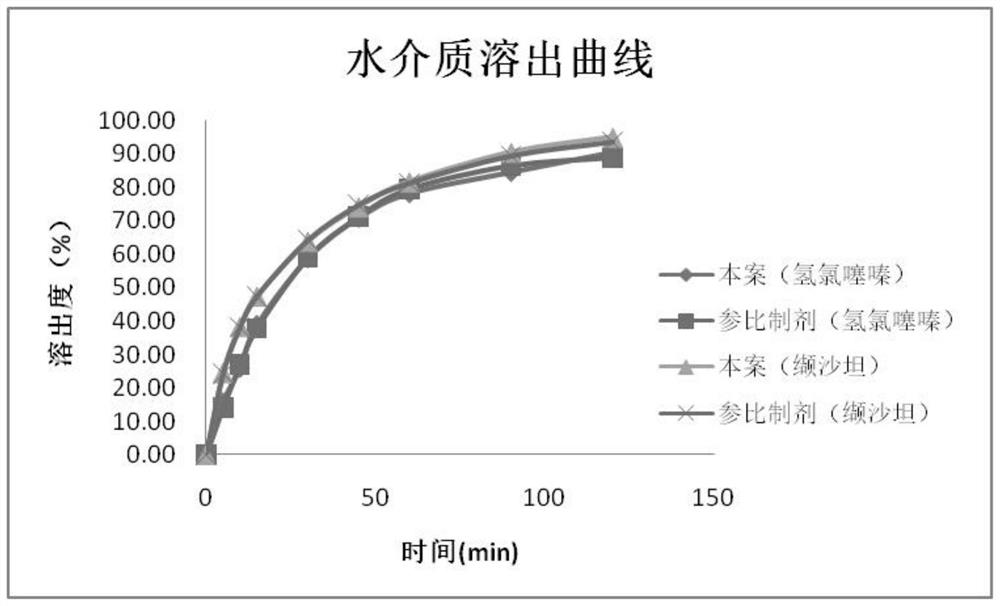
Embodiment 1
[0069] This embodiment provides a preparation process for a valsartan-hydrochlorothiazide compound preparation, comprising the following steps:
[0070] (1) Raw material pretreatment: the main drug valsartan and hydrochlorothiazide are respectively carried out to micronized pretreatment by jet mill;
[0071] (2) Excipient pretreatment: Povidone K30, poloxamer, sulfobutyl ether-β-cyclodextrin, low-substituted hypromellose, sodium lauryl sulfate, microcrystalline cellulose PH102 , crospovidone XL, magnesium stearate and colloidal silicon dioxide are passed through a 40-mesh sieve, and set aside;
[0072] (3) Preparation of dispersion: mix 35 parts of povidone K30, 62 parts of polyethylene glycol, 35 parts of mannitol, 4 parts of poloxamer, 5 parts of citric acid and 83 parts of valsartan and place under high pressure In the reactor, the temperature of the system in the autoclave was raised to 40°C; the supercritical CO 2 Send in the autoclave, control the pressure in the kettl...
Embodiment 2
[0088] This embodiment provides a preparation process for a valsartan-hydrochlorothiazide compound preparation, comprising the following steps:
[0089] (1) Raw material pretreatment: the main drug valsartan and hydrochlorothiazide are respectively carried out to micronized pretreatment by jet mill;
[0090] (2) Excipient pretreatment: Povidone K30, poloxamer, sulfobutyl ether-β-cyclodextrin, low-substituted hypromellose, sodium lauryl sulfate, microcrystalline cellulose PH102 , crospovidone XL, magnesium stearate and colloidal silicon dioxide are passed through a 40-mesh sieve, and set aside;
[0091] (3) Preparation of dispersion: mix 38 parts of povidone K30, 66 parts of polyethylene glycol, 45 parts of mannitol, 8 parts of poloxamer, 6 parts of citric acid and 90 parts of valsartan and place under high pressure In the reactor, the temperature of the system in the autoclave was raised to 40°C; the supercritical CO 2 Send in the autoclave, control the pressure in the kettl...
Embodiment 3
[0107] This embodiment provides a preparation process for a valsartan-hydrochlorothiazide compound preparation, comprising the following steps:
[0108] (1) Raw material pretreatment: the main drug valsartan and hydrochlorothiazide are respectively carried out to micronized pretreatment by jet mill;
[0109] (2) Excipient pretreatment: Povidone K30, poloxamer, sulfobutyl ether-β-cyclodextrin, low-substituted hypromellose, sodium lauryl sulfate, microcrystalline cellulose PH102 , crospovidone XL, magnesium stearate and colloidal silicon dioxide are passed through a 40-mesh sieve, and set aside;
[0110] (3) Preparation of dispersion: mix 40 parts of povidone K30, 65 parts of polyethylene glycol, 42 parts of mannitol, 6 parts of poloxamer, 6 parts of citric acid and 88 parts of valsartan and place under high pressure In the reactor, the temperature of the system in the autoclave was raised to 40°C; the supercritical CO 2 Send in the autoclave, control the pressure in the kettl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com