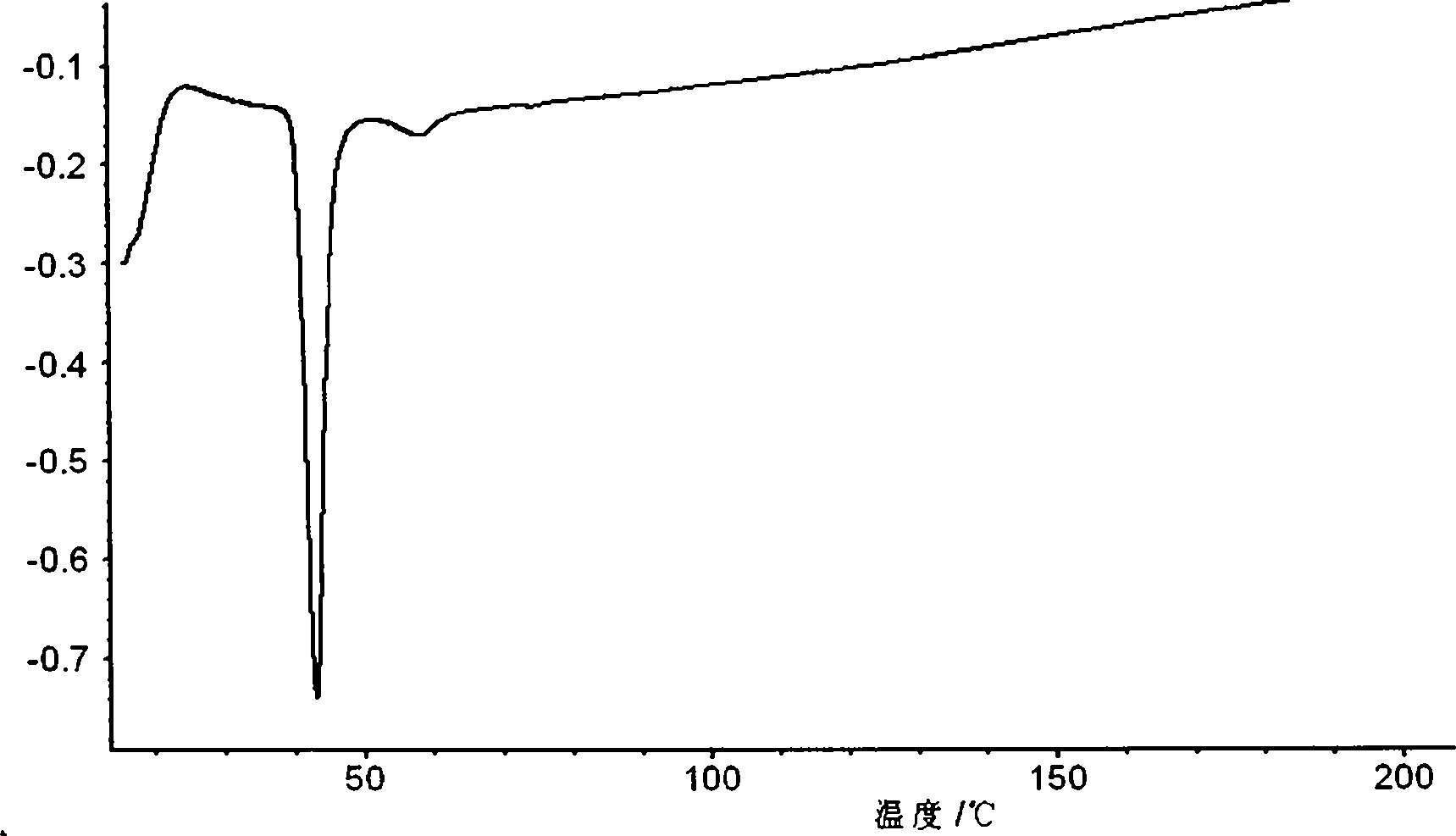
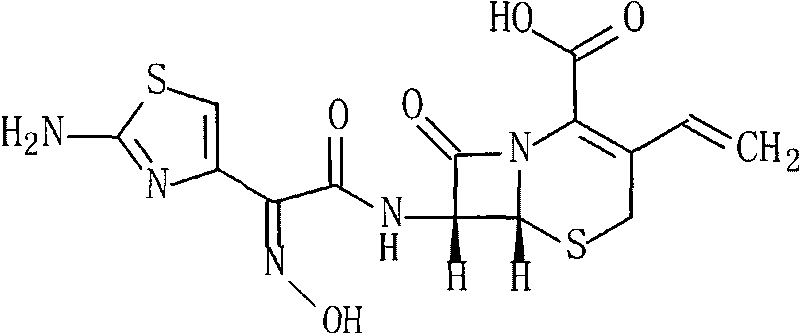
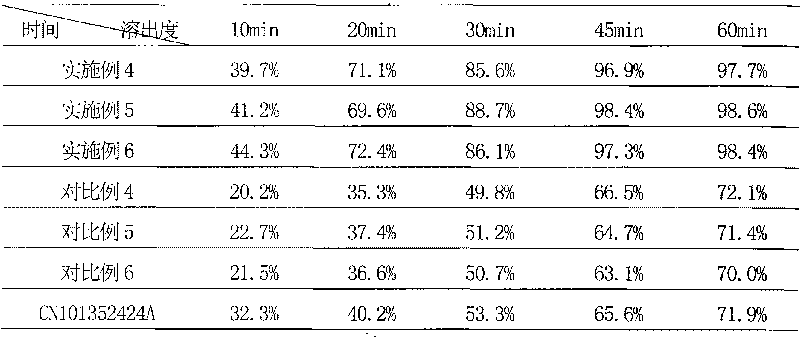
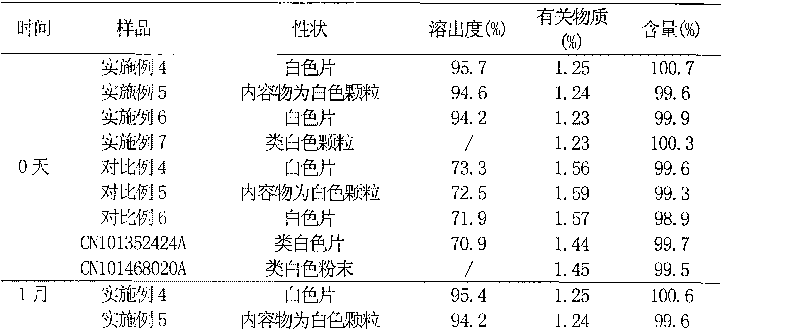
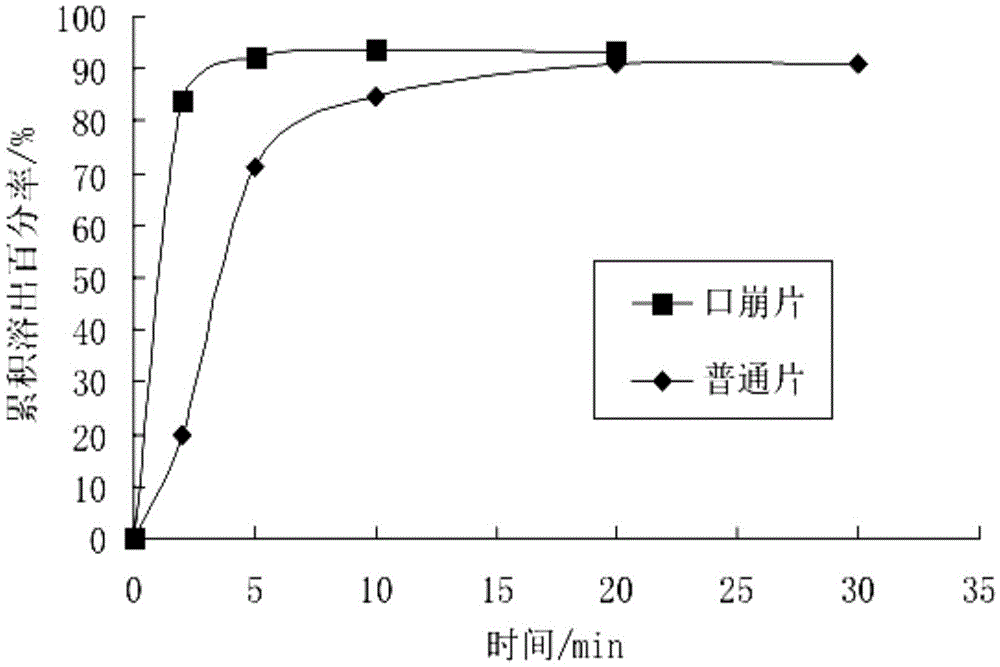
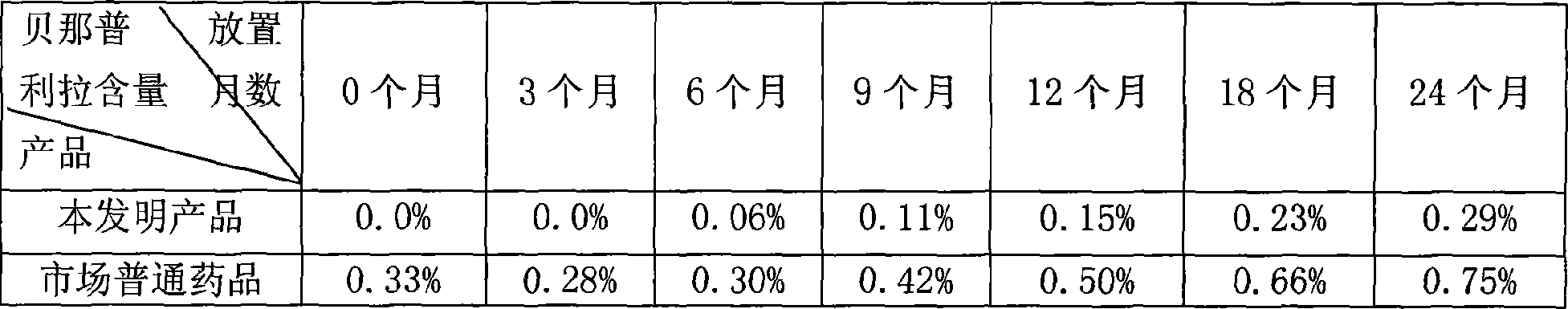
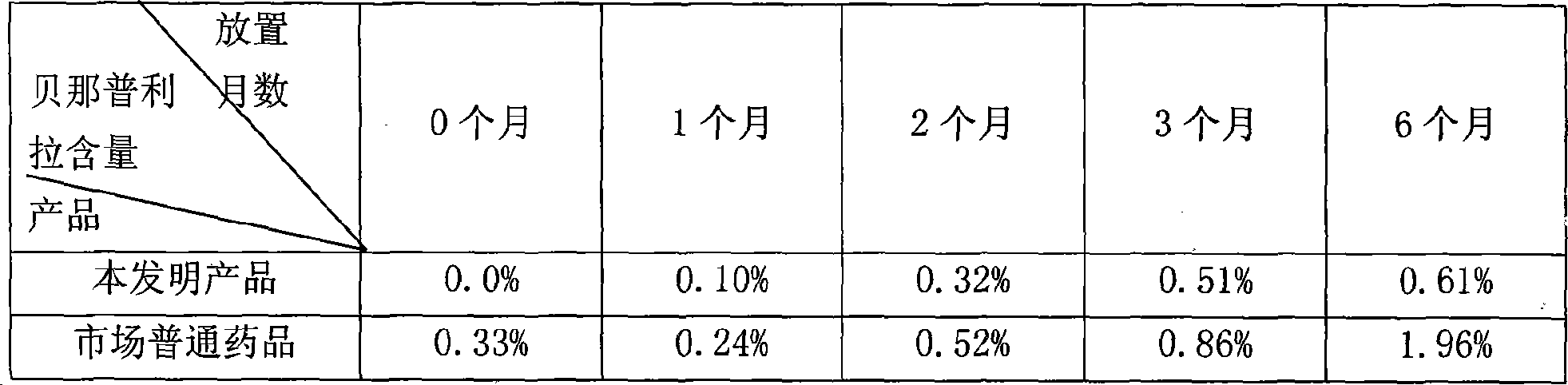
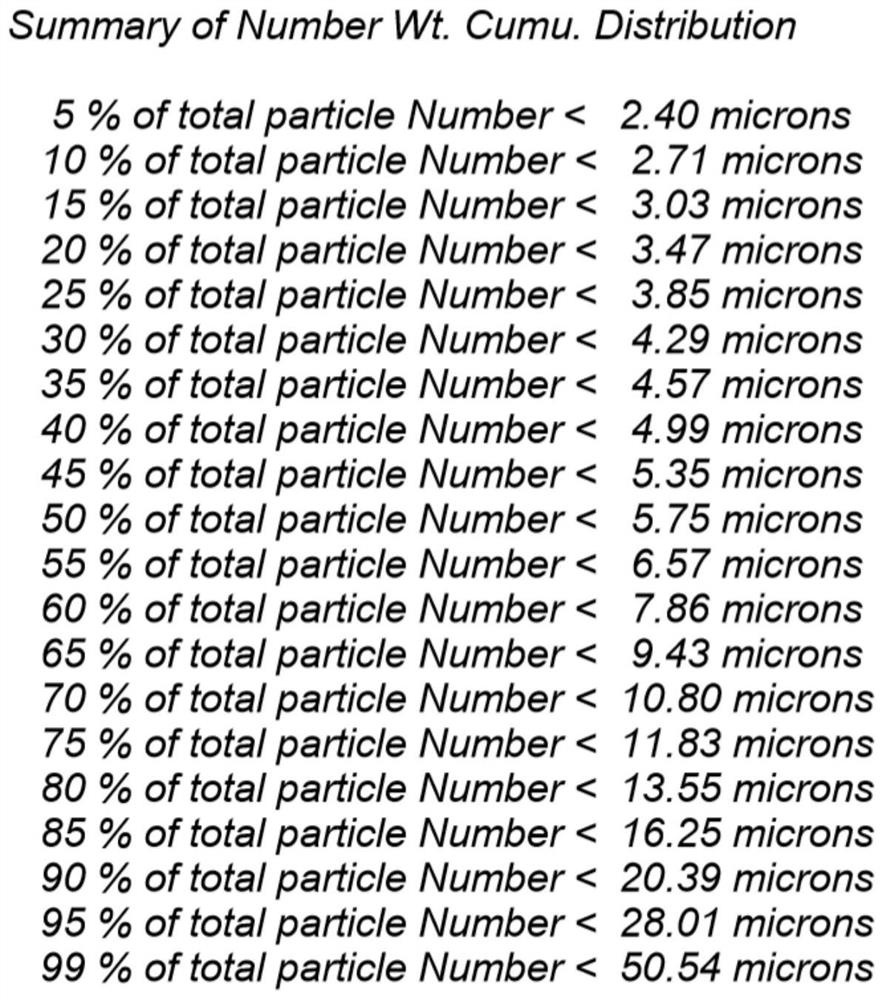
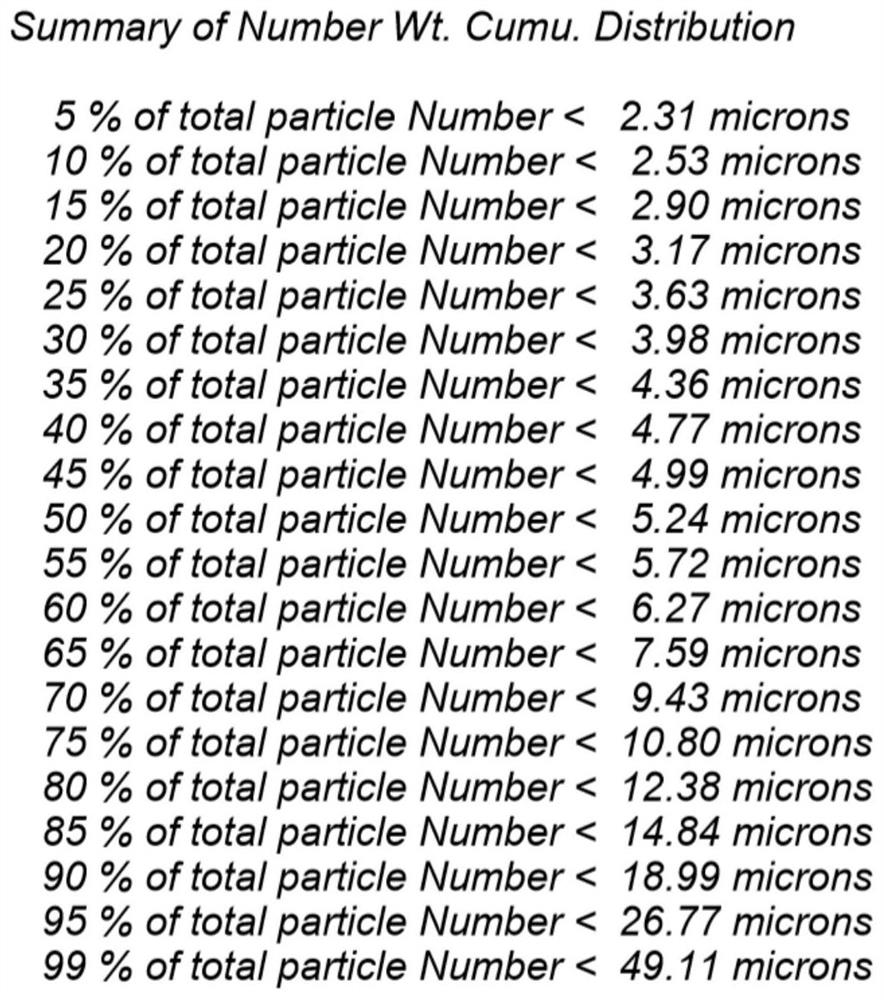
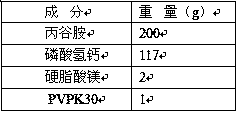
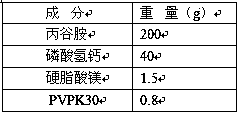
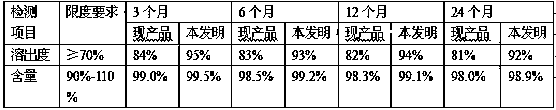
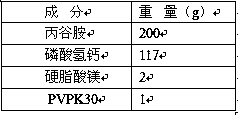
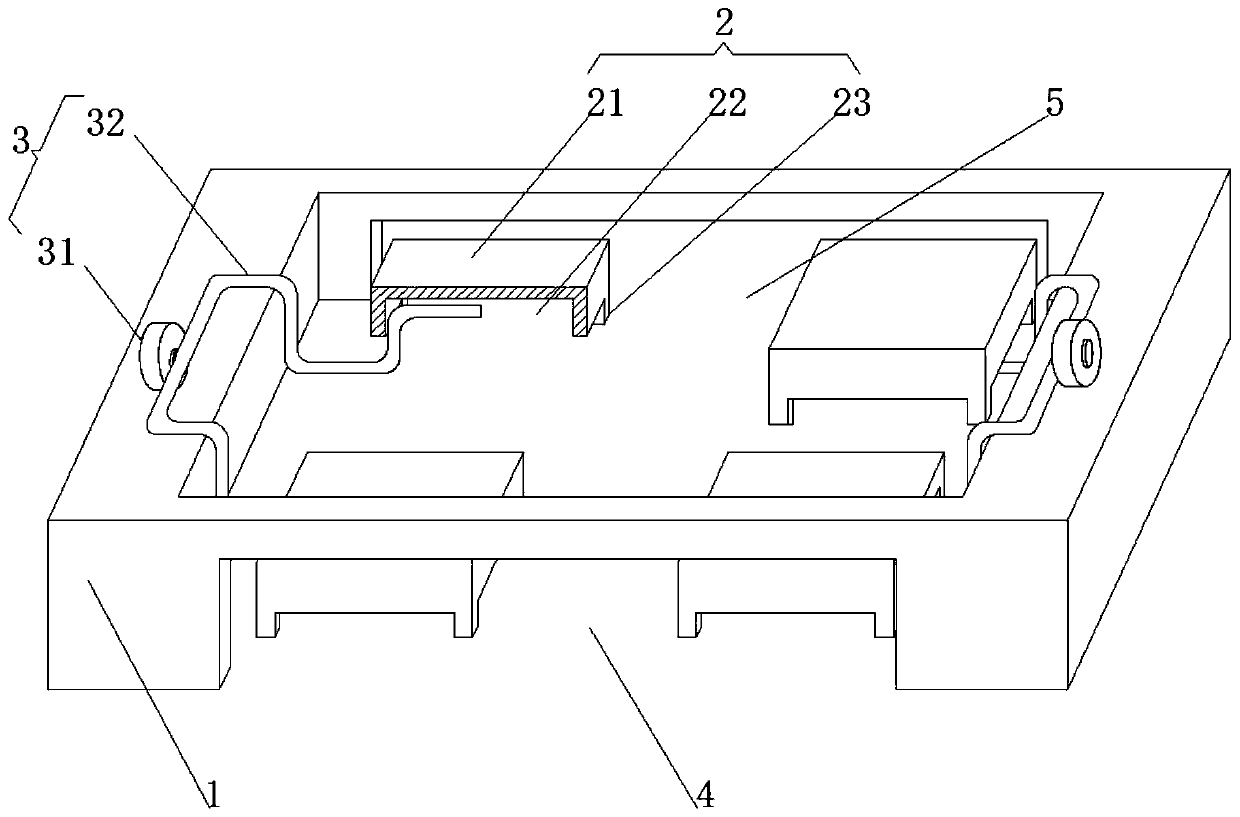
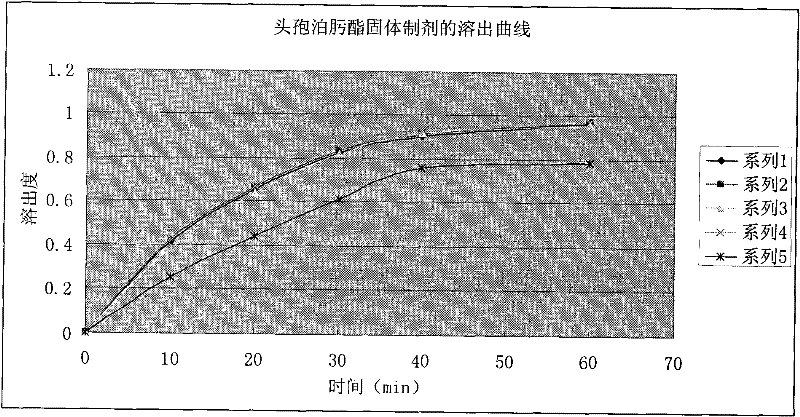
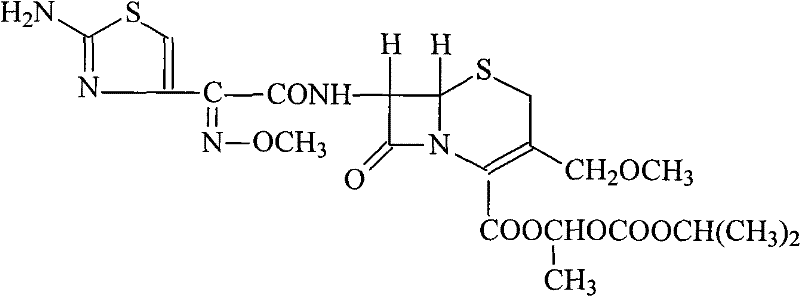
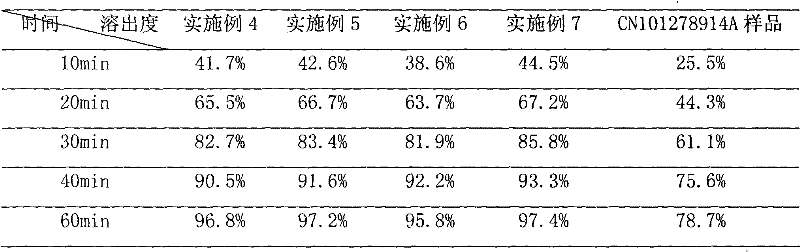
Patents
Literature
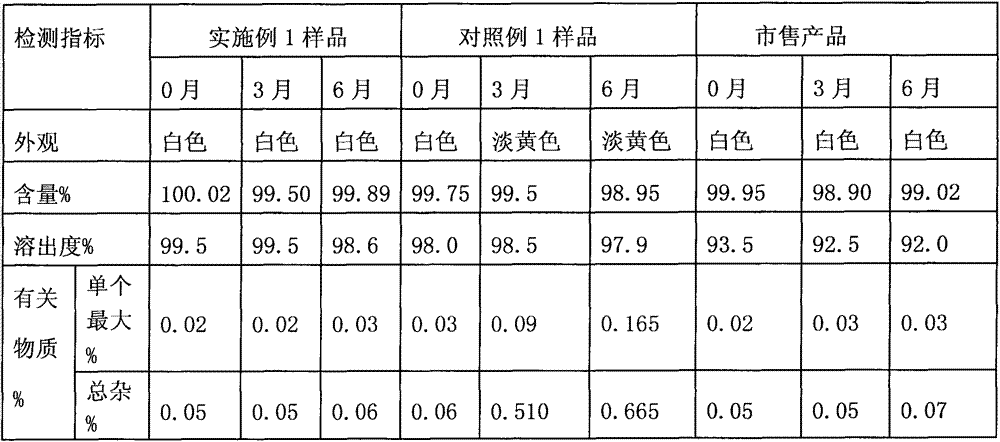
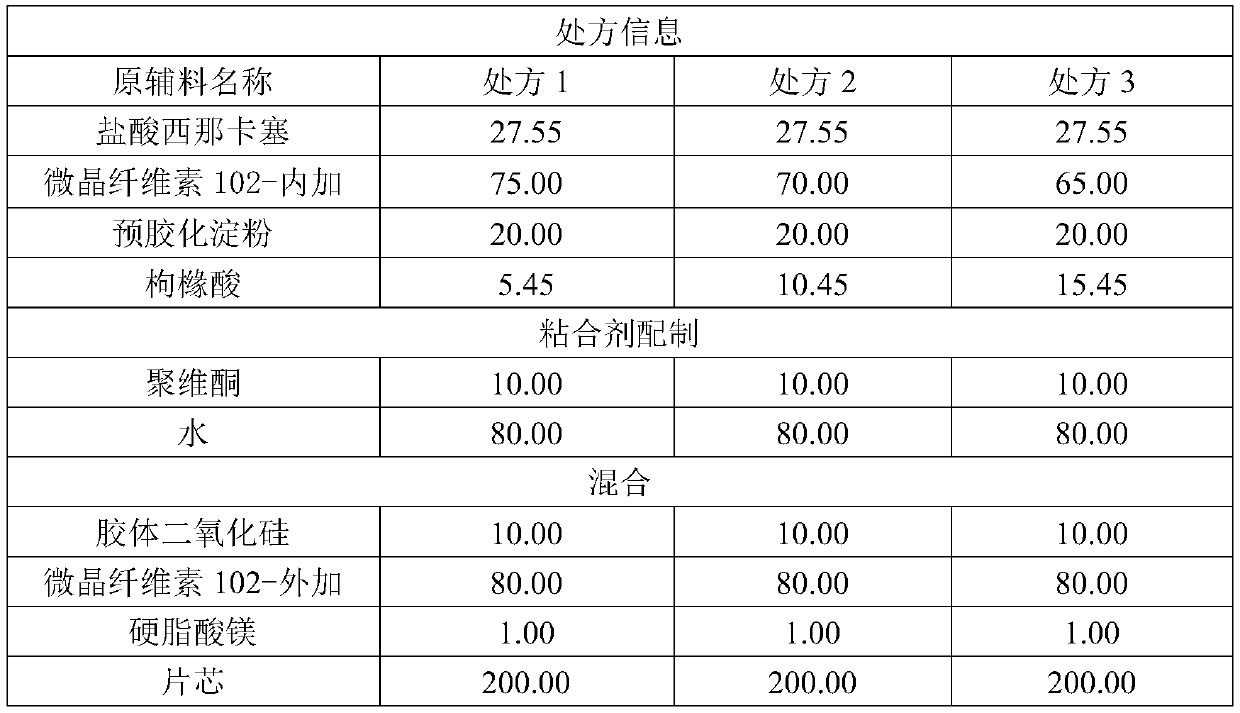
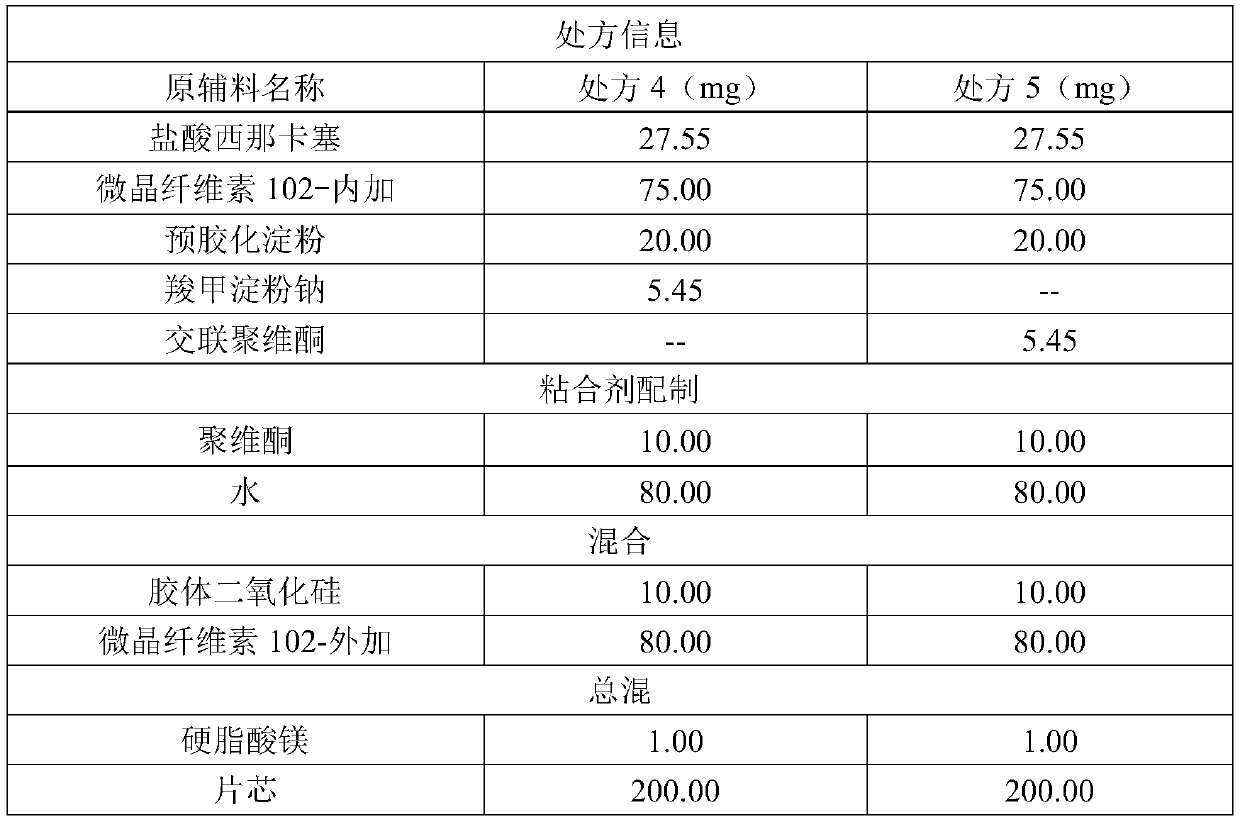
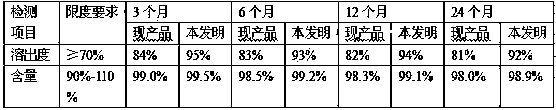
41results about How to "Solve the problem of low dissolution rate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
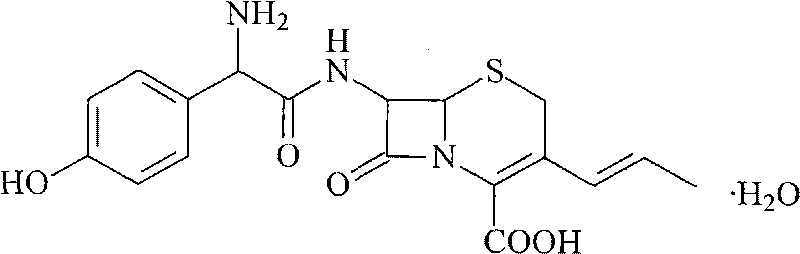
Cefprozil submicron emulsion solid preparation and new application thereof
InactiveCN101700232AImprove stabilityImprove solubilityAntibacterial agentsOrganic active ingredientsYolkEmulsion
The invention relates to a cefprozil submicron emulsion solid preparation and new application thereof in the preparation of medicine for treating acute plasma cell mastitis. The cefprozil submicron emulsion granule comprises cefprozil, yolk lecithin, poloxamer 188 and deoxysodium cholate with the weight ratio of 1:2.3-14:1.2-8:0.8-10.
Owner:HAINAN LINGKANG PHARMA CO LTD
Cefixime submicro-emulsion solid preparation and novel application thereof
InactiveCN101711741AImprove stabilityImprove solubilityAntibacterial agentsOrganic active ingredientsSolubilityEmulsion
The invention relates to a cefixime submicro-emulsion solid preparation and a novel application thereof, in particular to a solid preparation of cefixime processed by micro-emulsification and a novel application thereof. The cefixime submicro-emulsion solid preparation comprises cefixime, an emulsifying agent and an emulsifying aid agent, wherein the active component of the cefixime is processed by applying a micro-emulsification technology. The stability is enhanced, the solubility is increased, and the problem of low leaching degree is solved.
Owner:HAINAN MEIDA PHARMA
Celecoxib new formulation and preparation method thereof
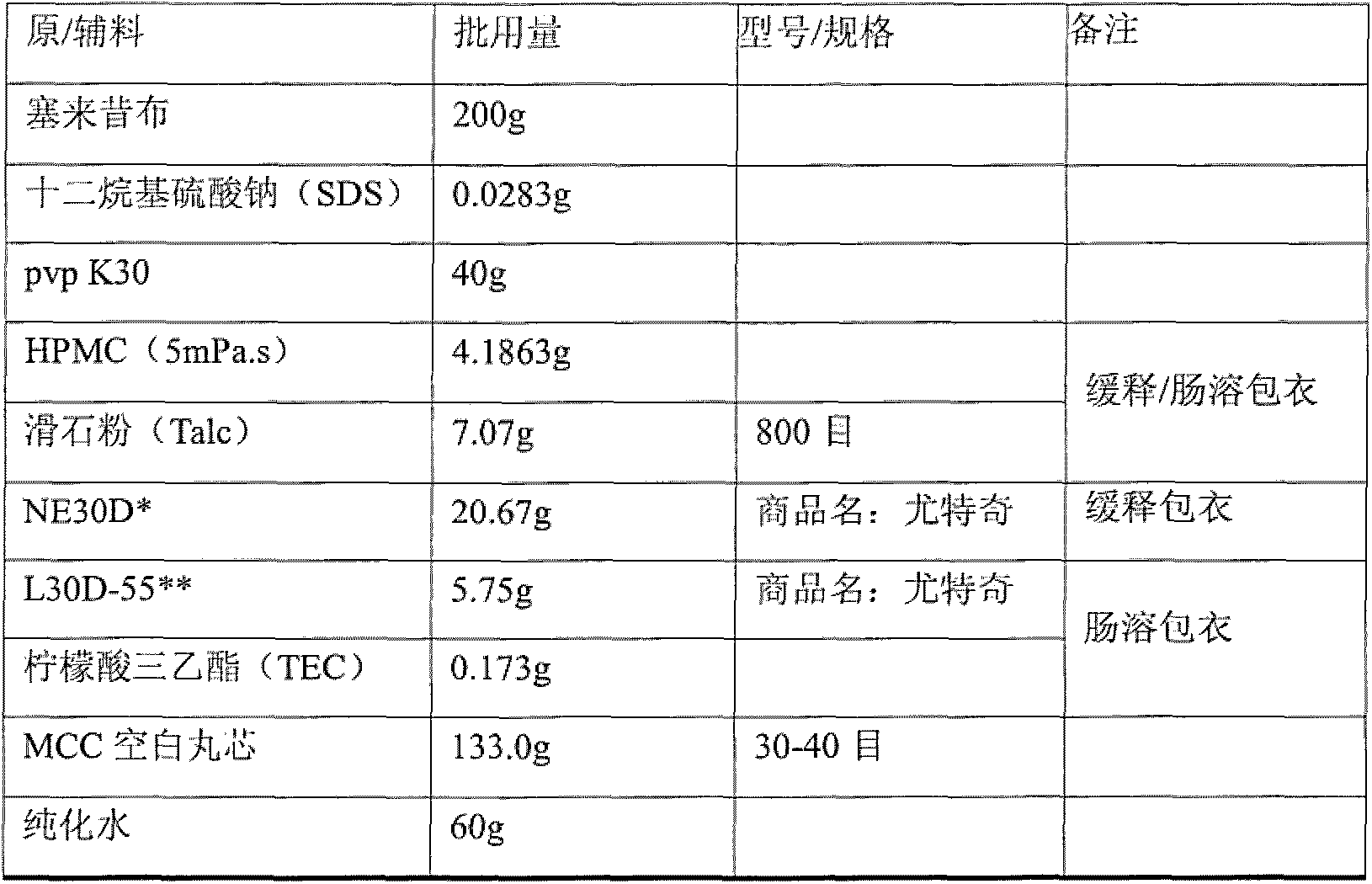
InactiveCN103191065ASolve the problem of low dissolution rateAccelerate dispersal and absorptionOrganic active ingredientsAntipyreticAdenocarcinoma polypsAnkylosing spondylitis
The invention discloses a celecoxib pellet preparation, which is used for treating rheumatic arthritis, osteoarthritis and ankylosing spondylitis, and also can be used for treating acute pain, dysmenorrheal, colorectal polyps, post-operation analgesia, low back pain, periarthritis of shoulder and tenosynovitis. According to the celecoxib new formulation and the preparation method thereof, the pellet preparation technology and the controlled-release pellet upper covering technology are adopted and microcrystalline cellulose pills are selected for dissolving the celecoxib material medicines in adhesive povidone solution, the medicine materials are uniformly sprayed on the surface of the pills and covered with isolating layers, so that the pellet is pressed and capsule. When the disintegration time limit of the celecoxib pellet preparation is remarkably shortened and the bioavailability is remarkably improved.
Owner:GUIZHOU LIANSHENG PHARMA
Orlistat oral preparation and preparation method thereof
InactiveCN103222964ASolve the problem of low dissolution rateWell mixedOrganic active ingredientsPowder deliveryAcrylic resinMedicine
The invention discloses an orlistat oral preparation and a preparation method thereof. The orlistat oral preparation is an orlistat tablet. The orlistat tablet comprises an orlistat acrylic resin solid dispersion, a disintegrating agent and pharmaceutically acceptable auxiliary materials. The orlistat acrylic resin solid dispersion is prepared by rapid expansion of supercritical solution and has D90 less than 10 microns. The raw materials of the orlistat oral preparation are micronized by a supercritical technology and an inert material layer is coated on the orlistat surface so that the problems of a low dissolution rate of the preparation obtained by the prior art, and sticking in tabletting are solved.
Owner:QINGDAO UNIV
Solid cefdinir sub-microemulsion and new application thereof
InactiveCN101721373AImprove stabilityImprove solubilityOrganic active ingredientsPill deliveryMicroemulsionCefdinir
The invention provides a solid cefdinir sub-microemulsion prepared from cefdinir sub-microemulsion particles and a pharmaceutically acceptable excipient and new application thereof. The cefdinir sub-microemulsion particle comprises the following components by weight part: 1 part of cefdinir, 2 to 15 parts of emulsifier and 0.5 to 9 parts of assistant emulsifier.
Owner:HAINAN MEIDA PHARMA
Method for preparing telmisartan and amlodipine double-layer tablets
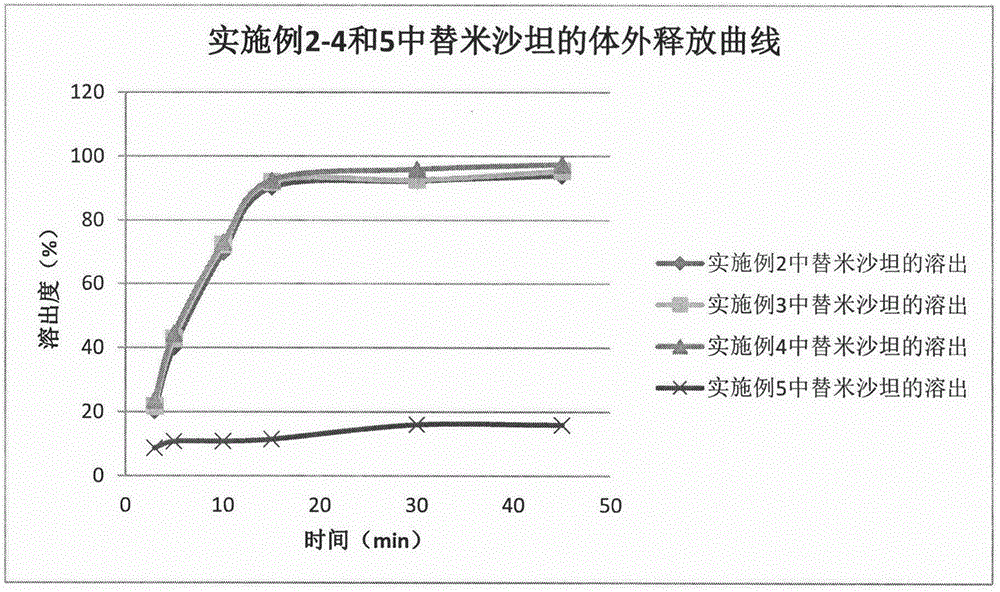
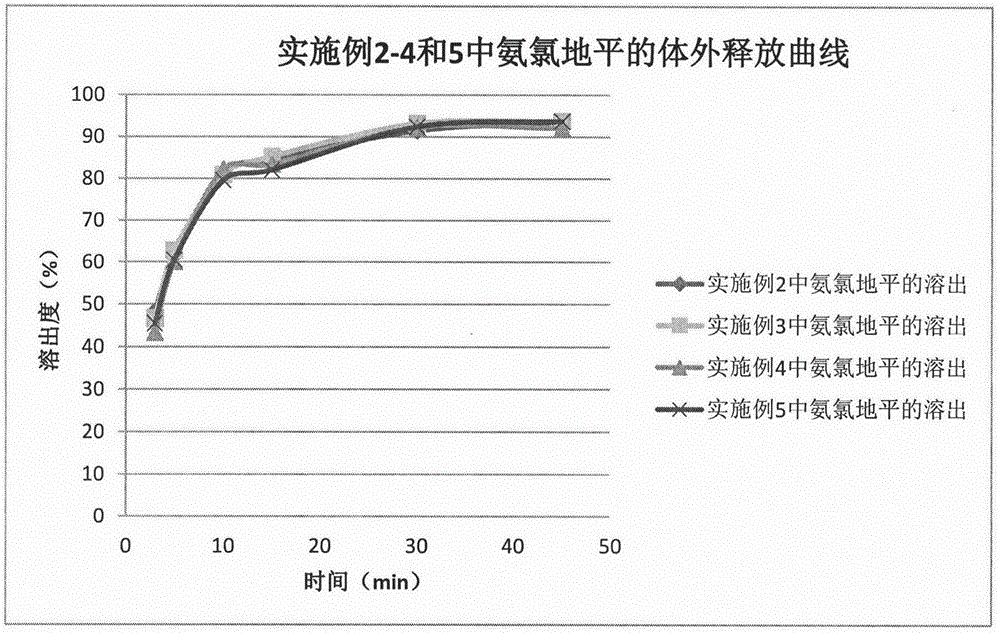
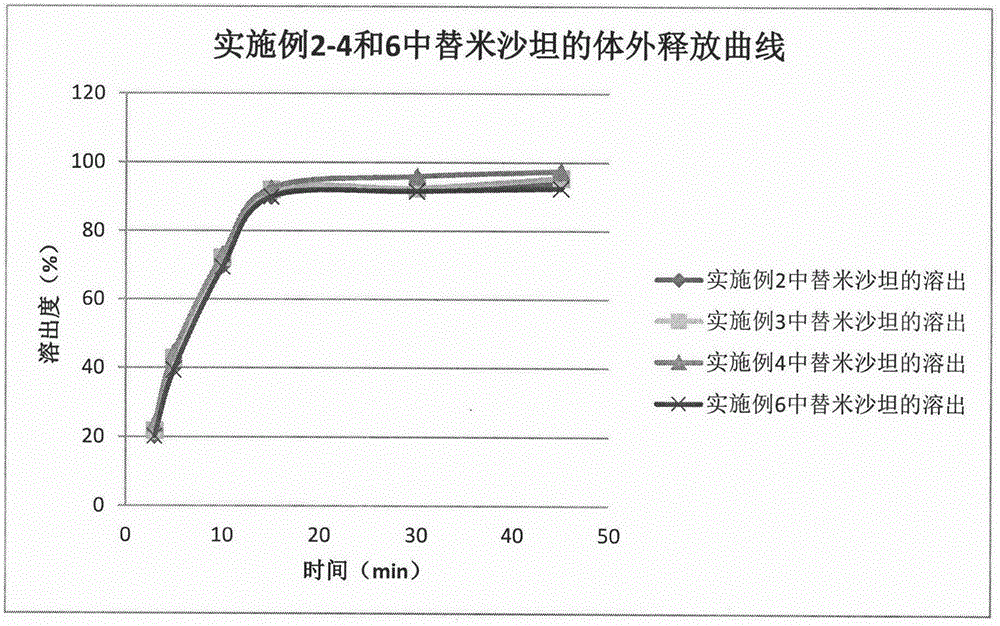
ActiveCN106822112ASolve the problem of low dissolution rateFix stability issuesOrganic active ingredientsPharmaceutical non-active ingredientsMedicineCurative effect
The invention discloses a method for preparing telmisartan and amlodipine double-layer tablets. The double-layer tablets comprise a telmisartan layer and an amlodipine besylate layer, wherein the telmisartan exists in a solid dispersion form. According to the method for preparing telmisartan and amlodipine double-layer tablets provided by the invention, good dissolution behaviors of the telmisartan and amlodipine besylate are guaranteed, and high stability of the amlodipine besylate is also guaranteed, so that the quality and curative effects of the telmisartan and amlodipine double-layer tablets are ensured. The preparation method is simple and feasible and is suitable for industrial production.
Owner:JIANGSU YABANG AIPUSEN PHARMA
Infantile domperidone orally disintegrating tablet and preparation method thereof
ActiveCN105560199AGreat tasteAddressing AdherenceOrganic active ingredientsDigestive systemCrospovidonesOrally disintegrating tablet
The invention discloses an infantile domperidone orally disintegrating tablet and a preparation method thereof. The orally disintegrating tablet is prepared from the following raw materials in parts by mass: 10 to 30 parts of domperidone, 10 to 30 parts of fatty glyceride, 5 to 10 parts of tween 80, 900 to 2700 parts of white sugar, 2 to 5 parts of crospovidone, 10 to 30 parts of mannitol, 0.01 to 0.05 part of essence, 0.2 to 0.5 part of magnesium stearate and 0.2 to 0.5 part of aerosil. According to the infantile domperidone orally disintegrating tablet and the preparation method thereof, an orally disintegrating tablet medicament is prepared by using the domperidone as an effective component of the medicament and through an innovative process. By using the domperidone orally disintegrating tablet prepared by the invention, the problems that the dissolution rate of the medicament is low, the medicament has a bitter taste, and the like, are solved successfully; the infantile domperidone orally disintegrating tablet has a favorable effect on infantile functional dyspepsia.
Owner:SHANDONG SBOND PHARMA +1
Benazepril pharmaceutical compsn. and process for its prepn.
InactiveCN101032491ASolve the problem of low dissolution rateAvoid degradationPowder deliveryPill deliveryProcess equipmentMedicine
The present invention provides one kind of medicine composition of benazepril and its preparation process. The present invention solves the problem of benazepril with low dissolution and the problems of the preparation process in medicine sticking and medicine degradation. The preparation process combines solid dispersing technology and traditional tablet preparation, and is simple, accurate in dosage control and high in production efficiency.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD
Canagliflozin composition
InactiveCN107744512ASolve the problem of low dissolution rateHigh dissolution rateOrganic active ingredientsMetabolism disorderMedicineSodium citrate
The invention relates to a Canagliflozin tablet composition, and belongs to the technical field of medicine preparations. The technical scheme is that the unit dose of Canagliflozin composition is prepared from 100 to 300 mg of canagliflozin with D90 being 36 to 60 micrometers, 40 to 70 mg of lactose, 0.8 to 1.5 mg of lauryl sodium sulfate, 7 to 15 mg of sodium citrate, 3 to 6 mg of sodium carbonate, 10 to 18 mg of beta-cyclodextrin, 3 to 8 mg of povidone K30, 3 to 8 mg of polyvinylpolypyrrolidone and 1 to 1.5mg of magnesium stearate. The technical scheme provides the Canagliflozin tablet composition with the advantages of high dissolution rate and stability.
Owner:WEIHAI GUANBIAO INFORMATION TECH
Preparation method for microcrystalline cellulose capable of increasing drug dissolution rate
The invention discloses a preparation method for microcrystalline cellulose capable of increasing the drug dissolution rate and belongs to the technical field of pharmacy. The preparation method for the microcrystalline cellulose capable of increasing the drug dissolution rate comprises the following steps that raw materials are put into a reaction still containing acid liquor, a steam valve is opened to heat up, and a temperature reaction is maintained; water is added into a storage tank, materials after the reaction in step (1) are added into the storage tank, and still standing is conducted after even stirring to enable the materials to be layered; the upper layer thick liquid of the material part after still standing is extracted for 1 / 4-3 / 4 and then subjected to centrifugal washing, after the thick liquid is spin-dried and washed till the pH value of water outflowing from an water outlet of a centrifugal machine is 6-7, washing is stopped, centrifuge dripping is continuous, and a material A is obtained; the material A after centrifuge dripping is dried, the dried material A is obtained; and pulverizing and sieving are conducted, specifically, the dried material A obtained from the last step is evenly added into a pulverizer to be pulverized, and microcrystalline cellulose A, namely microcrystalline cellulose capable of improving the drug dissolution rate, is prepared after pulverizing and sieving. According to the preparation method for the microcrystalline cellulose capable of increasing the drug dissolution rate, the defect that the dissolution rate of traditional microcrystalline cellulose for an indissolvable drug is low and unstable is overcome.
Owner:QUFU TIANLI MEDICAL SUPPLEMENTS CO LTD
Valsartan and hydrochlorothiazide compound preparation and preparation process thereof
ActiveCN113041250AImprove solubilityImprove bioavailabilityPharmaceutical product form changePharmaceutical non-active ingredientsValsartanAdhesive
The invention discloses a valsartan and hydrochlorothiazide compound preparation and a preparation process thereof. The preparation process comprises the following steps: pretreating raw materials; pretreating auxiliary materials; preparing dispersion: dispersing valsartan on the surface of the carrier by adopting a supercritical fluid impregnation technology; preparing inclusion compound: carrying out inclusion on hydrochlorothiazide by adopting sulfobutyl ether-beta-cyclodextrin; preparing pre-coated particles: conducting top spraying granulation on the hydrochlorothiazide inclusion compound through a composite adhesive; premixing: mixing the valsartan solid dispersion, the auxiliary materials and the hydrochlorothiazide pre-coated particles; distributing materials: dividing the premixed powder into two parts; respectively carrying out dry granulation on the two parts of premixed powder; mixing totally; tabletting; and coating to obtain the product. The compound preparation with good bioavailability and drug stability is prepared by combining a supercritical impregnation technology, a secondary inclusion technology and a different oil pressure powder granulation technology, the production cost is low, and the process is simple and easy to implement.
Owner:上海耀大生物科技有限公司
High-dissolubility fenofibrate tablet and preparation method thereof
InactiveCN107126420AHigh dissolution rateGood dissolution effectOrganic active ingredientsMetabolism disorderDocosahexaenoic acidMedicine
The invention discloses a high-dissolubility fenofibrate tablet and a preparation method thereof. The high-dissolubility fenofibrate tablet is prepared by coating a fenofibrate raw material with docosahexaenoic acid-beta-cyclodextrin, performing spray-drying and mixing and directly tabletting with auxiliary materials. By adopting the preparation method disclosed by the invention, the problems that by using a conventional method, the dissolubility is low and the bioavailability is poor as the mass of medicines is hard to control are solved; and compared with a conventional direct tabletting and wet-method pelleting process, the preparation method has the advantages that the dissolubility of medicines is effectively improved, the medicines are relatively good in dissolubility effect and the bioavailability is improved.
Owner:SHANGHAI SINE WANXIANG PHARMA +1
Cefixime submicro-emulsion solid preparation and novel application thereof
InactiveCN101711741BImprove stabilityImprove solubilityAntibacterial agentsOrganic active ingredientsSolubilityEmulsion
The invention relates to a cefixime submicro-emulsion solid preparation and a novel application thereof, in particular to a solid preparation of cefixime processed by micro-emulsification and a novel application thereof. The cefixime submicro-emulsion solid preparation comprises cefixime, an emulsifying agent and an emulsifying aid agent, wherein the active component of the cefixime is processed by applying a micro-emulsification technology. The stability is enhanced, the solubility is increased, and the problem of low leaching degree is solved.
Owner:HAINAN MEIDA PHARMA
Danazol tablet composition
InactiveCN108785264ASmooth appearanceHigh dissolution rateOrganic active ingredientsPharmaceutical non-active ingredientsCarboxymethyl starchSulfate
The invention relates to a danazol tablet composition, and belongs to the technical field of medicine preparations. The danazol tablet composition in a unit dose comprises 100 mg of danazol, 1.4-2.6 mg of lecithin, 1.2-1.8 mg of sodium lauryl sulfate, 36-64 mg of lactose, 8-15 mg of low-substituted hydroxypropyl methyl celluloses, 4-8 mg of sodium carboxymethyl starch and 1.0-1.8 mg of magnesium stearate. The danazol tablet composition has the advantage that the clinical requirements can be met by the danazol tablet composition.
Owner:WEIHAI GUANBIAO INFORMATION TECH
Stainless steel matte finishing process
The invention belongs to the technical field of surface treatment of medical equipment and relates to a stainless steel matte finishing process. The primary technical scheme is as follows: the stainless steel matte finishing process comprises the following steps: placing a deoiled stainless steel workpiece and the anode of a power supply connected in an anodizing matte finishing solution of a mixture containing 10-50 g / L H3PO4, 10-30 g / L C6H8O7, 100-300 g / L H2O2 and 0.4-1 g / L K2SO4; heating the solution to 60-80 DEG C and connecting a platinum and titanium net to the cathode of a direct current power supply, wherein the area ratio of the platinum and titanium net and the cathode of the direct current power supply is 1: 1; and keeping the distance between the cathode and the anode at 6.0 cm, carrying out anodizing for 30-60 min under the condition that the current density is 3.4-6.8 A / dm<2> and the working voltage is 9.4-10.5 V, wherein the surface of stainless steel workpiece is matteand has silver metal gloss, the hardness of the stainless steel is improved, and the pitting corrosion resistance is improved. The stainless steel matte finishing process provided by the invention notonly can solve the environmental problem that an existing stainless steel matte finishing solution contains harmful substances and strong acids, but also can solve the problem that the dissolving speed of the environment-friendly solution is low, thereby providing a new path for development of stainless steel matte technical treatment.
Owner:CHONGQING UNIVERSITY OF SCIENCE AND TECHNOLOGY
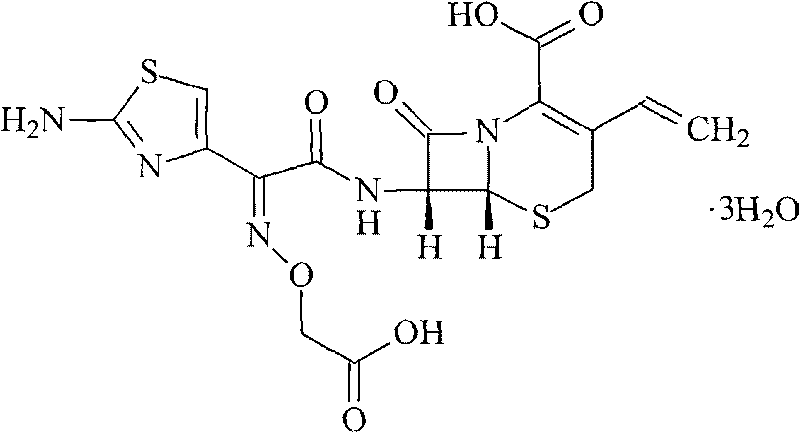
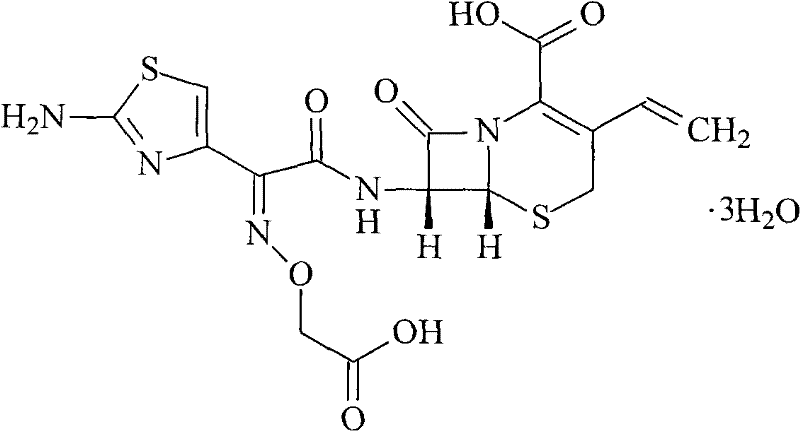
Method for improving dissolution rate of cefquinome sulfate and method for detecting dissolution rate of cefquinome sulfate
InactiveCN111714501ASolve the problem of low dissolution rateImprove drug dissolutionAntibacterial agentsOrganic active ingredientsCEFQUINOME SULFATEDissolution
The invention discloses a method for improving the dissolution rate of cefquinome sulfate and a method for detecting the dissolution rate of the cefquinome sulfate. The method comprises the followingsteps of 1) mixing soybean oil for injection and ethyl oleate according to a ratio of 5: 5, heating mixed liquid to 120 DEG C under the protection of N2, keeping the temperature for 2 hours, and cooling the liquid to the room temperature; 2) heating the mixed liquid to 60 DEG C, adding BHT, performing stirring and melting, adding hydrogenated castor oil and span 60 when the liquid is cooled to 40DEG C, performing stirring for 30 minutes, and performing cooling to the room temperature; and 3) adding a prescription amount of the cefquinome sulfate after all medicinal auxiliary materials are added, pouring the cefquinome sulfate into a colloid mill, and performing grinding for 5-20 minutes in a manner of alternating circulating grinding and non-circulating grinding. The particle size of thecefquinome sulfate is changed through the colloid mill, so that the drug dissolution rate of the cefquinome sulfate is increased; and through particle size detection and dissolution rate analysis, after grinding is conducted for 15-20 min, the cefquinome sulfate with the particle size of 10 microns or below accounts for 80%, and the dissolution rate is the best.
Owner:杭州爱力迈动物药业有限公司
Agomelatine oral liquid preparation as well as preparation method and application thereof
ActiveCN106692039AGood water solubilitySolve the problem of low dissolution rateOrganic active ingredientsNervous disorderAdjuvantMass ratio
The invention discloses an Agomelatine oral liquid preparation as well as a preparation method and application thereof. The Agomelatine oral liquid preparation comprises Agomelatine, cyclodextrin and a pharmaceutically-acceptable adjuvant, wherein the mass ratio of Agomelatine to cyclodextrin is (1:1) to (1:50). The oral liquid is in a stable and uniform aqueous solution state, solves the problem of low dissolution rate of an Argomeline tablet, ensures the rapid and uniform absorption of the Agomelatine in body gastrointestinal tracts and reduces the individual absorption difference of the Argomeline. The oral liquid preparation disclosed by the invention is less in adjuvant dosage, simple in preparation process, low in cost and suitable for large-scale production. Therefore, the developed oral liquid has significant market values and prospects.
Owner:ANCUREALL PHARMA SHANGHAI CO LTD
Composition
ActiveCN103610661ASolve the sticking problemSolve the problem of low dissolution rateOrganic active ingredientsMetabolism disorderCross-linkBenzoic acid
The invention relates to an alogliptin benzoate tablet composition. The alogliptin benzoate tablet composition is characterized by comprising 34 g of alogliptin benzoate, 2 to 12 g of silicon dioxide, 30 to 77.5 g of mannitol, 25 to 62.5 g of microcrystalline cellulose, 6 g of cross-linked sodium carboxymethyl cellulose, 4 g of hydroxypropylcellulose, 1.5 g of magnesium stearate and a suitable amount of opadry coating powder in every 1,000 tablets. Through reasonable compatibility and by the adoption of a direct pressing process, the production process is simplified, the production time is shortened, the production efficiency is improved and the product stability is improved compared with the conventional wet granulation process.
Owner:DISHA PHARMA GRP
a composition
ActiveCN103610661BSolve the sticking problemSolve the problem of low dissolution rateOrganic active ingredientsMetabolism disorderMANNITOL/SORBITOLCroscarmellose sodium
The invention relates to a tablet composition containing alogliptin benzoate. It is characterized in that every 1000 tablets contain alogliptin benzoate 34g, silicon dioxide 2-12g, mannitol 30-77.5g, microcrystalline cellulose 25-62.5g, croscarmellose sodium 6g, hydroxyl Propylene cellulose 4g, magnesium stearate 1.5g, Opadry coating powder appropriate amount. Through reasonable compatibility, the present invention adopts the direct compression process, which simplifies the production process, shortens the production time, improves the production efficiency and improves the product stability compared with the traditional wet granulation process.
Owner:DISHA PHARMA GRP
Pitavastatin calcium composition
InactiveCN105213339ASolve the problem of uneven contentSolve the problem of low dissolution rateMetabolism disorderPill deliveryPitavastatineSodium carboxymethylcellulose
Owner:DISHA PHARMA GRP
Medicine composition containing cinacalcet hydrochloride and production method of medicine composition containing cinacalcet hydrochloride
PendingCN111450073ASimple preparation processLow costOrganic active ingredientsMetabolism disorderSodium bicarbonatePharmaceutical drug
The invention discloses a medicine composition containing cinacalcet hydrochloride and a production method of the medicine composition containing the cinacalcet hydrochloride. The medicine compositioncontaining the cinacalcet hydrochloride is mainly characterized in that accessories of an effervescent disintegrant, such as citric acid, tartaric acid or sodium bicarbonate, and another pharmaceutically-acceptable excipient, such as a binder, a filler or a lubricant, are used, wet granulation is adopted, the production technology is simplified, the production cost is reduced, and produced tablets have the advantages that the appearance is excellent, dissolution is good, the stability is high, internal absorption is good, and the like.
Owner:FUJIAN HAIXI PHARMA
Proglumide pharmaceutical composition
ActiveCN105213336ASolve the problem of low dissolution ratePromote dissolutionOrganic active ingredientsDigestive systemProglumideHydrogen phosphate
The invention discloses a proglumide pharmaceutical composition. The pharmaceutical composition is characterized in that each 1000 tablets contain the following components in percentage by weight: 190-210 parts of proglumide, 40-210 parts of calcium hydrogen phosphate, 1-5 parts of magnesium stearate and 0-5 parts of a binding agent. A preparation method of the composition comprises the following steps. The pharmaceutical composition disclosed by the invention has advantages that the calcium hydrogen phosphate, which is alkaline, serves as an accessory material, and the proglumide is slightly soluble in water but is solution in an alkaline solution. By virtue of the alkaline accessory material, various quality indexes of a finished product are kept basically stable within a valid period, and moreover the dissolution rate of the product is improved. The calcium hydrogen phosphate, which serves an accessory material, can accelerate disintegration of the finished product while addition of a disintegrating agent, so that cost of the finished product can be reduced.
Owner:KAMP PHARMA
A kind of proglumide pharmaceutical composition
ActiveCN105213336BPromote oral absorptionSolve the problem of low dissolution rateOrganic active ingredientsDigestive systemHydrogen phosphateMagnesium stearate
Owner:KAMP PHARMA
Nilotinib tablet composition
InactiveCN108653223ASolve the problem of low dissolution rateSolve the problem of reduced dissolution rate during storageOrganic active ingredientsPharmaceutical non-active ingredientsCrospovidonesDissolution
The invention relates to a nilotinib composition, belonging to the technical field of pharmacy. The nilotinib composition is characterized in that a unit dose of the composition is prepared from 200mgof nilotinib, 36-60mg of chitosan, 36-52mg of lactose, 7-10mg of crospovidone, 4-8mg of alginic acid, 1-3mg of fumed silica, 0.8-1.6mg of sodium dodecyl sulfate and 1.3-2.6mg of magnesium stearate. The stable nilotinib tablet composition with a high dissolution rate can be obtained.
Owner:WEIHAI YUNRUI INFORMATION TECH CO LTD
A stainless steel matt treatment process
The invention belongs to the technical field of surface treatment of medical equipment and relates to a stainless steel matte finishing process. The primary technical scheme is as follows: the stainless steel matte finishing process comprises the following steps: placing a deoiled stainless steel workpiece and the anode of a power supply connected in an anodizing matte finishing solution of a mixture containing 10-50 g / L H3PO4, 10-30 g / L C6H8O7, 100-300 g / L H2O2 and 0.4-1 g / L K2SO4; heating the solution to 60-80 DEG C and connecting a platinum and titanium net to the cathode of a direct current power supply, wherein the area ratio of the platinum and titanium net and the cathode of the direct current power supply is 1: 1; and keeping the distance between the cathode and the anode at 6.0 cm, carrying out anodizing for 30-60 min under the condition that the current density is 3.4-6.8 A / dm<2> and the working voltage is 9.4-10.5 V, wherein the surface of stainless steel workpiece is matteand has silver metal gloss, the hardness of the stainless steel is improved, and the pitting corrosion resistance is improved. The stainless steel matte finishing process provided by the invention notonly can solve the environmental problem that an existing stainless steel matte finishing solution contains harmful substances and strong acids, but also can solve the problem that the dissolving speed of the environment-friendly solution is low, thereby providing a new path for development of stainless steel matte technical treatment.
Owner:CHONGQING UNIVERSITY OF SCIENCE AND TECHNOLOGY
Ambrisentan tablet composition
InactiveCN109276546ASolving Content Uniformity ProblemsSolve the problem of low dissolution rateOrganic active ingredientsInorganic non-active ingredientsLactoseDissolution
The invention relates to an Ambrisentan tablet composition, and belongs to the technical field of pharmacy. Per thousand tablets of Ambrisentan tablet composition comprise 5 to 10g of Ambrisentan, 32to 50g of lactose, 6 to 12g of calcium hydrophosphate, 16 to 28g of superfine silica gel powder, 28 to 50g of microcrystalline cellulose, 1.5 to 4g of croscarmellose sodium and 0.8 to 1.6g of magnesium stearate. The invention provides the qualified Ambrisentan tablet composition; and the problems of low dissolution rate and low content uniformity of the tablet in the preparation process are solved.
Owner:WEIHAI GUANBIAO INFORMATION TECH
Orlistat oral preparation and preparation method thereof
InactiveCN103222964BSolve the problem of low dissolution rateWell mixedOrganic active ingredientsPowder deliveryOrlistatAcrylic resin
The invention discloses an orlistat oral preparation and a preparation method thereof. The orlistat oral preparation is an orlistat tablet. The orlistat tablet comprises an orlistat acrylic resin solid dispersion, a disintegrating agent and pharmaceutically acceptable auxiliary materials. The orlistat acrylic resin solid dispersion is prepared by rapid expansion of supercritical solution and has D90 less than 10 microns. The raw materials of the orlistat oral preparation are micronized by a supercritical technology and an inert material layer is coated on the orlistat surface so that the problems of a low dissolution rate of the preparation obtained by the prior art, and sticking in tabletting are solved.
Owner:QINGDAO UNIV
Orally disintegrating domperidone tablet for children and preparation method thereof
ActiveCN105560199BGreat tasteAddressing AdherenceOrganic active ingredientsDigestive systemCrospovidonesMANNITOL/SORBITOL
The invention discloses an infantile domperidone orally disintegrating tablet and a preparation method thereof. The orally disintegrating tablet is prepared from the following raw materials in parts by mass: 10 to 30 parts of domperidone, 10 to 30 parts of fatty glyceride, 5 to 10 parts of tween 80, 900 to 2700 parts of white sugar, 2 to 5 parts of crospovidone, 10 to 30 parts of mannitol, 0.01 to 0.05 part of essence, 0.2 to 0.5 part of magnesium stearate and 0.2 to 0.5 part of aerosil. According to the infantile domperidone orally disintegrating tablet and the preparation method thereof, an orally disintegrating tablet medicament is prepared by using the domperidone as an effective component of the medicament and through an innovative process. By using the domperidone orally disintegrating tablet prepared by the invention, the problems that the dissolution rate of the medicament is low, the medicament has a bitter taste, and the like, are solved successfully; the infantile domperidone orally disintegrating tablet has a favorable effect on infantile functional dyspepsia.
Owner:SHANDONG SBOND PHARMA +1
Water treatment aeration tank
InactiveCN110776094AIncrease contact areaExtension of timeSpecific water treatment objectivesBiological treatment apparatusSewageWater treatment
The invention discloses a water treatment aeration tank. The water treatment aeration tank comprises a tank body, gas storage tanks are fixedly mounted in the tank body, air inlet units are fixedly mounted in the tank body, each gas storage tank comprises a tank body, a groove and support columns, the tank body is fixedly mounted in the tank body through the support columns, the groove is disposedat the middle of a space below the tank body, each air inlet unit comprises a draught fan and a pipeline, one end of the pipeline is fixedly connected to an air outlet end of the draught fan, the other end of the pipeline is connected to the upper part inside the groove, three or more gas storage tanks and air inlet units are disposed in the tank body, the top view of the groove body is rectangular or circular, and a water inlet is arranged on one side of the tank body. The water treatment aeration tank is designed through the structures of the gas storage tanks and the air inlet units, the contact area and contact time of air and sewage in the aeration tank is increased, also the sewage boosts the air, and the dissolution rate of oxygen in the air in the sewage is greatly increased.
Owner:苏州奥辰机械设备有限公司
Cefpodoxime proxetil submicron emulsion solid preparation and novel application thereof
InactiveCN101708166BImprove stabilityImprove solubilityAntibacterial agentsOrganic active ingredientsEmulsionBioavailability
The invention discloses a cefpodoxime proxetil submicron emulsion solid preparation and a novel application thereof, particularly a cefpodoxime proxetil solid preparation which is subjected to micro-emulsification and a novel application thereof. In the invention, the micro-emulsification technology is applied to process cefpodoxime proxetil raw materials so as to obtain a cefpodoxime proxetil submicron emulsion with excellent performance, the stability of the cefpodoxime proxetil is improved and the dissolution rate of the cefpodoxime proxetil preparation is obviously improved, so that the cefpodoxime proxetil submicron emulsion solid preparation has better bioavailability and can be used for preparing a medicament for treating osteomyelitis of jaws.
Owner:HAINAN MEIDA PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com