a composition
A composition and benzoic acid technology, applied in the field of medicine, can solve the problems of slow tablet disintegration, increased tablet substance, poor solubility, etc., and achieve the effects of solving low dissolution rate, improving fluidity, and solving poor fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
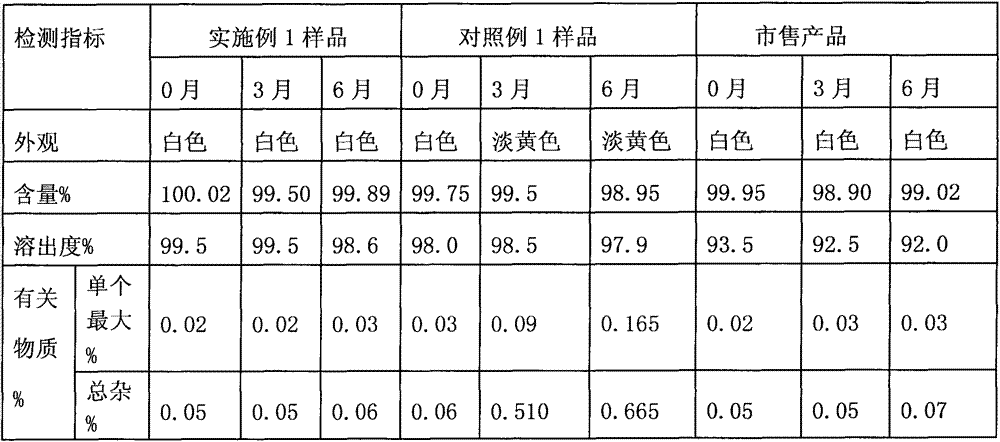
Image
Examples
Embodiment 1
[0020] Embodiment 1, prescription: alogliptin benzoate 34g, silicon dioxide 12g, mannitol 30g, microcrystalline cellulose 62.5g, croscarmellose sodium 6g, hydroxypropyl cellulose 4g, stearic acid Magnesium 1.5g. Prepare 1000 tablets according to the preparation method described in the technical scheme, and coat the plain tablets with 10% Opadry 50% ethanol solution.
Embodiment 2
[0021] Embodiment 2, prescription: alogliptin benzoate 34g, silicon dioxide 2g, mannitol 77.5g, microcrystalline cellulose 25g, croscarmellose sodium 6g, hydroxypropyl cellulose 4g, stearic acid Magnesium 1.5g. Prepare 1000 tablets according to the preparation method described in the technical scheme, and coat the plain tablets with 10% Opadry 50% ethanol solution.
Embodiment 3
[0022] Embodiment 3, prescription: alogliptin benzoate 34g, silicon dioxide 6g, mannitol 50g, microcrystalline cellulose 48.5g, croscarmellose sodium 6g, hydroxypropyl cellulose 4g, stearic acid Magnesium 1.5g. Prepare 1000 tablets according to the preparation method described in the technical scheme, and coat the plain tablets with 10% Opadry 50% ethanol solution.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com