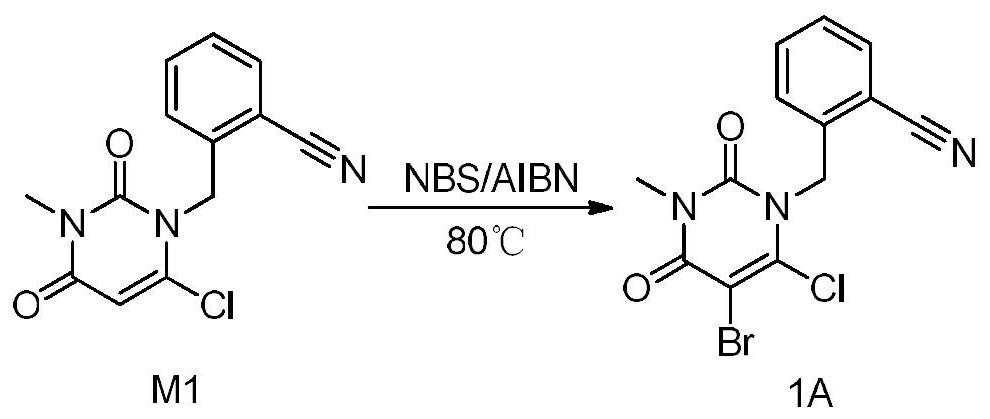
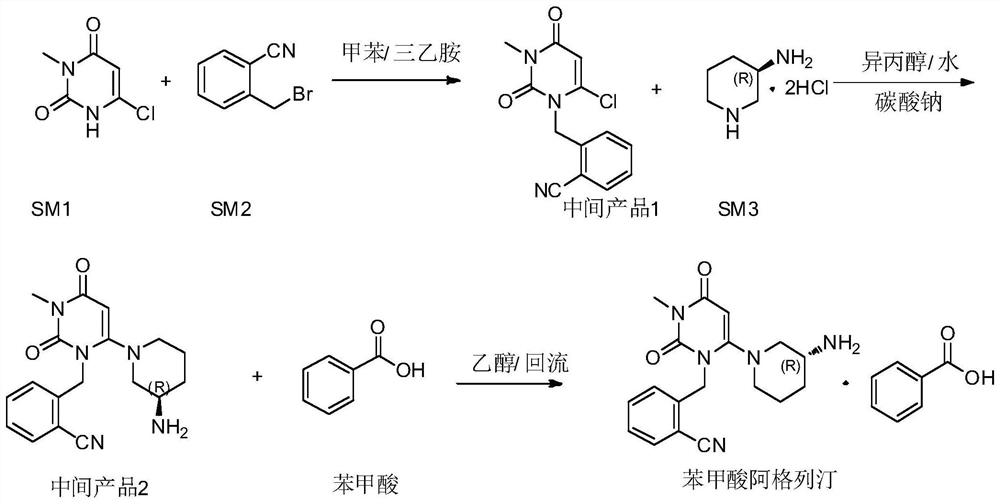
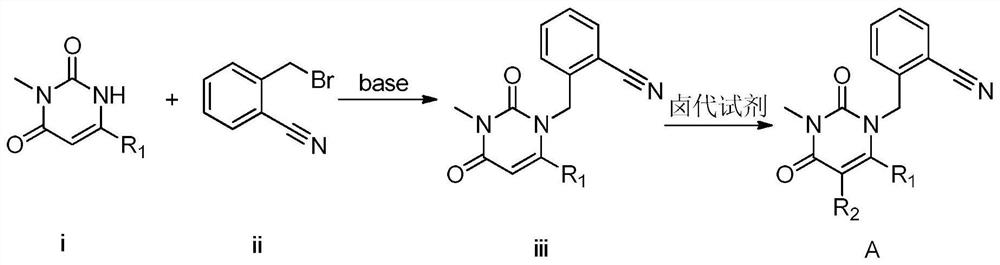
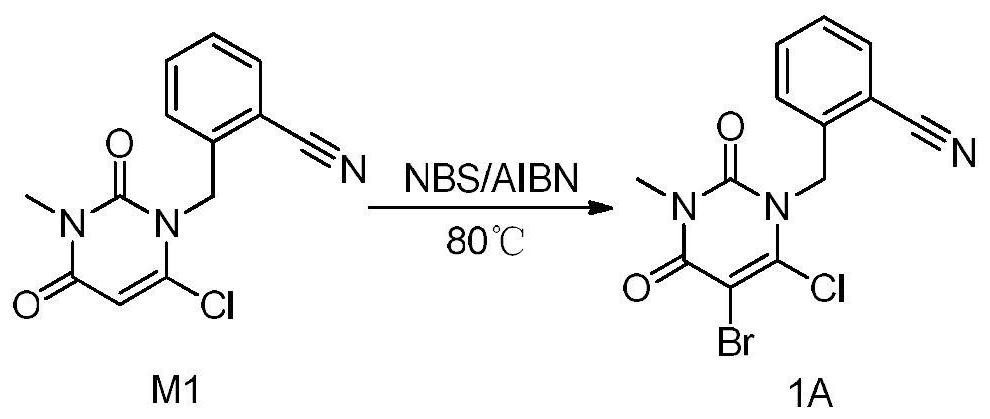
Patents
Literature
52 results about "Alogliptin Benzoate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
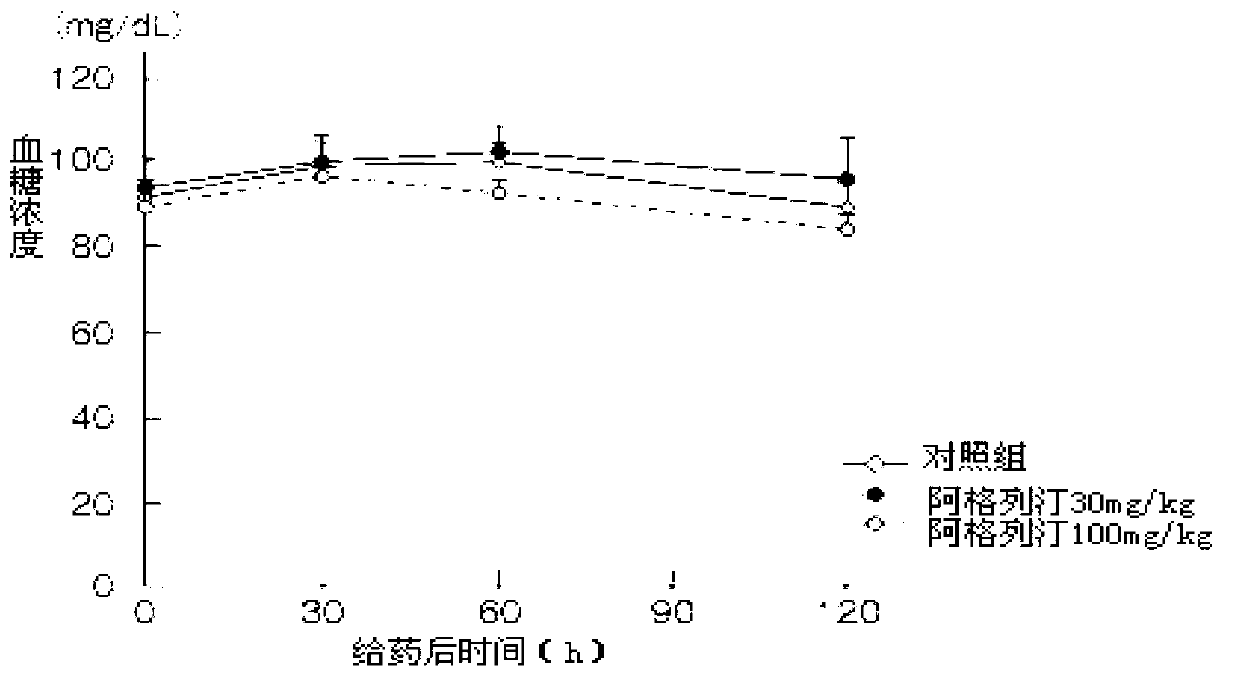
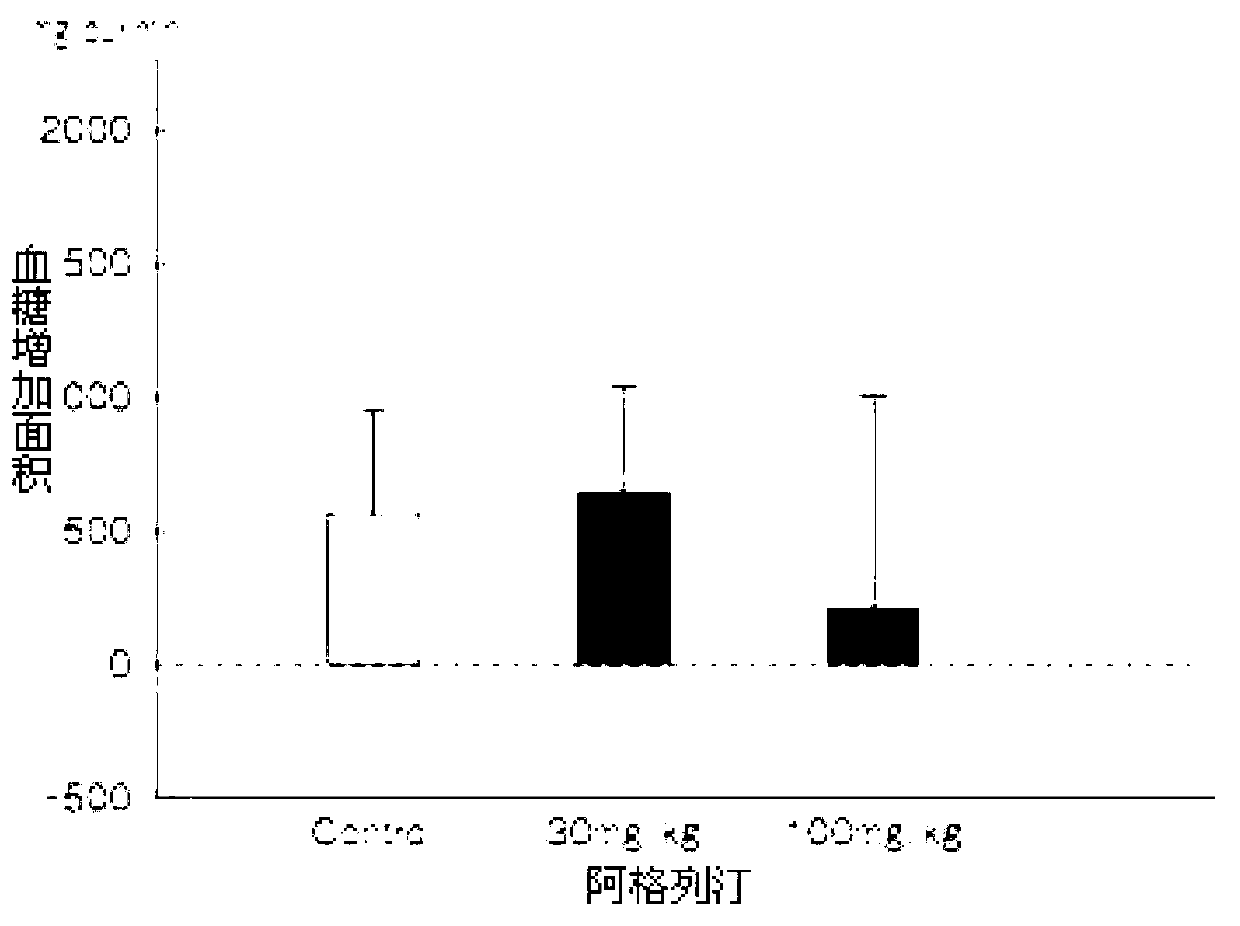
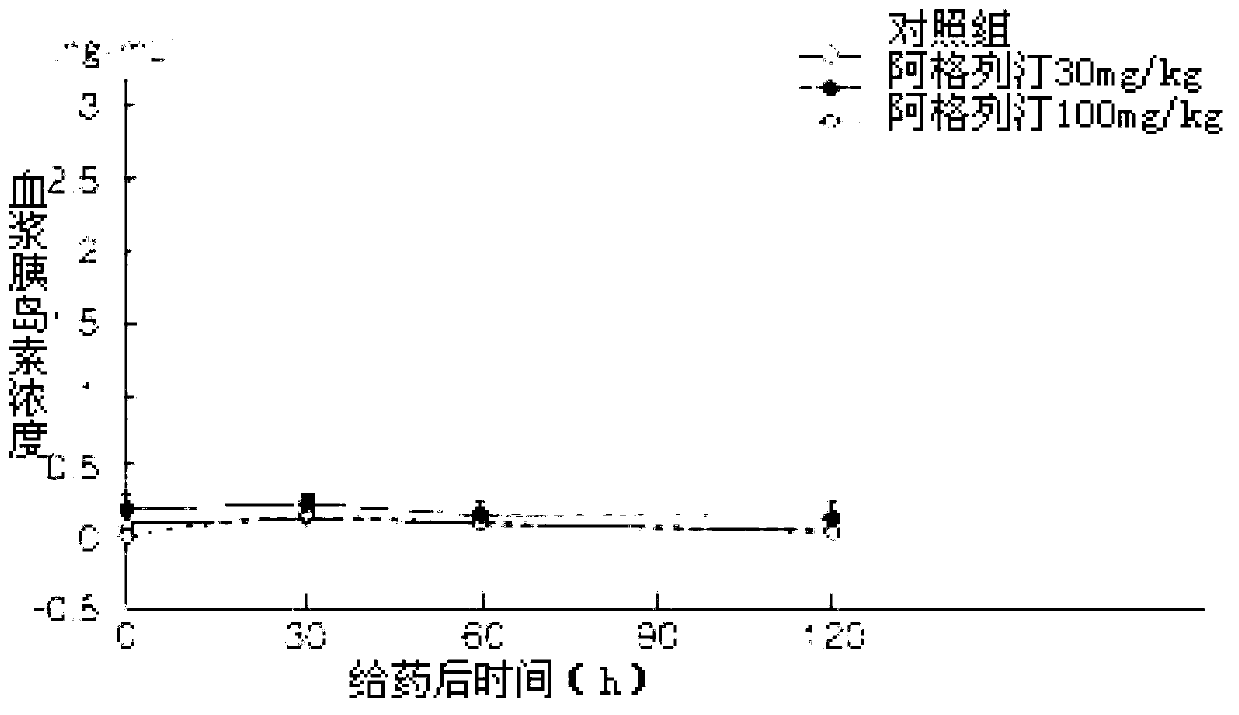
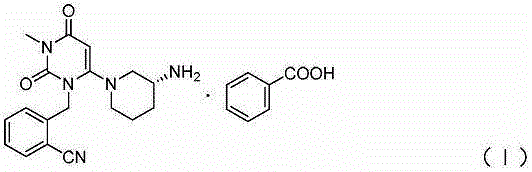
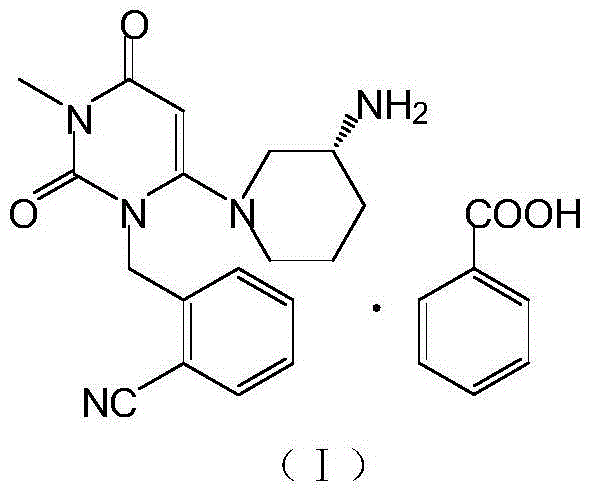
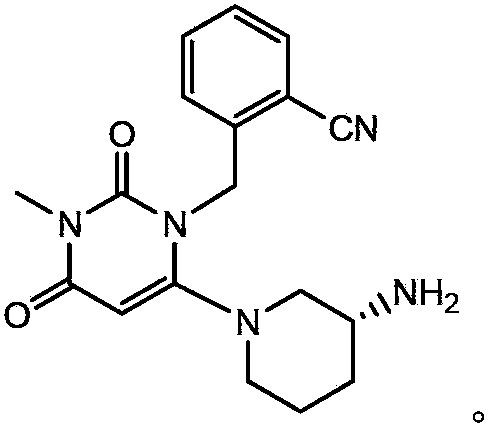
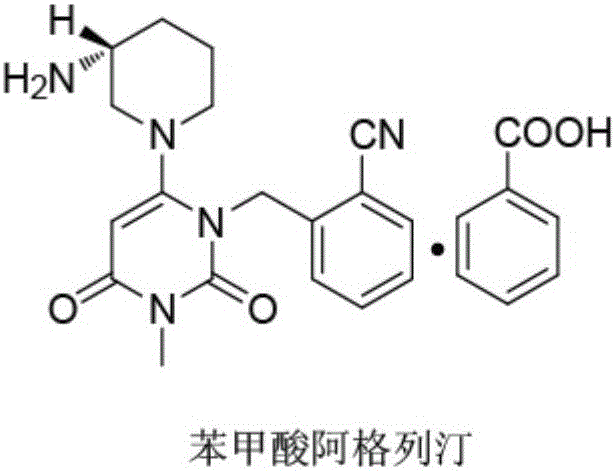
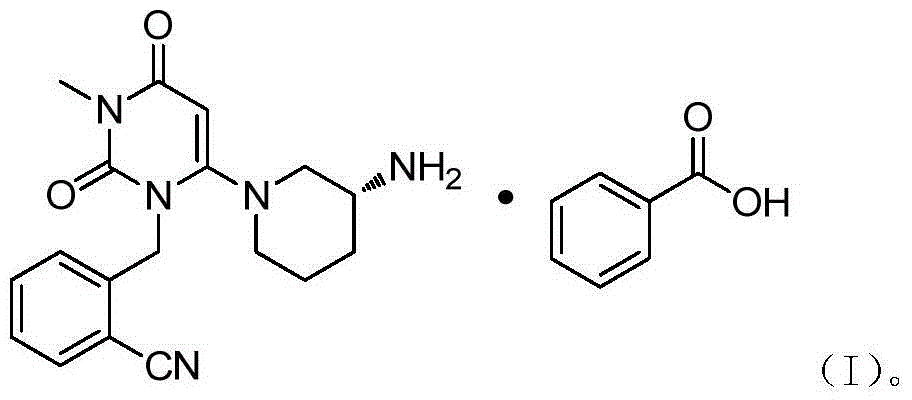
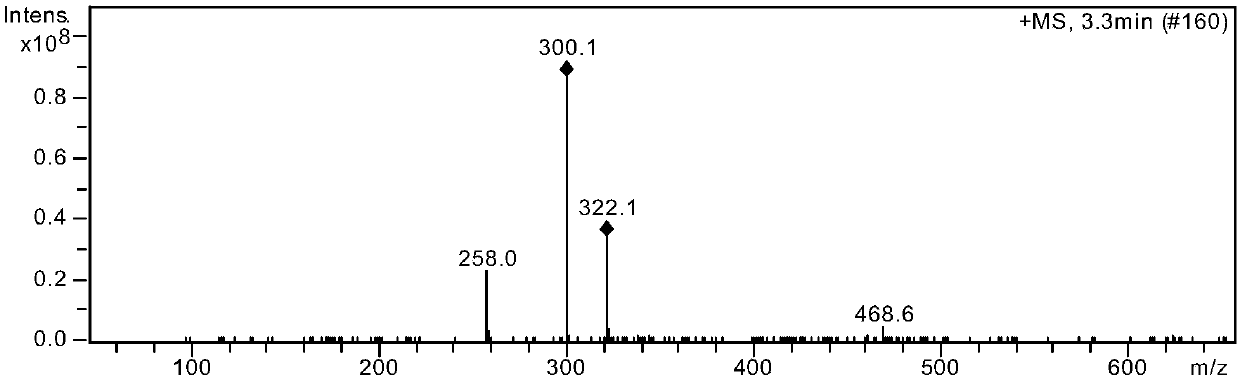
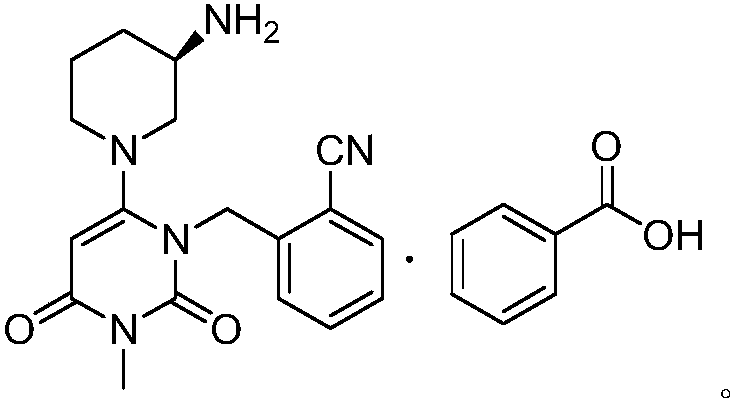
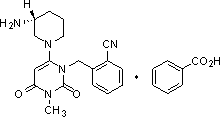
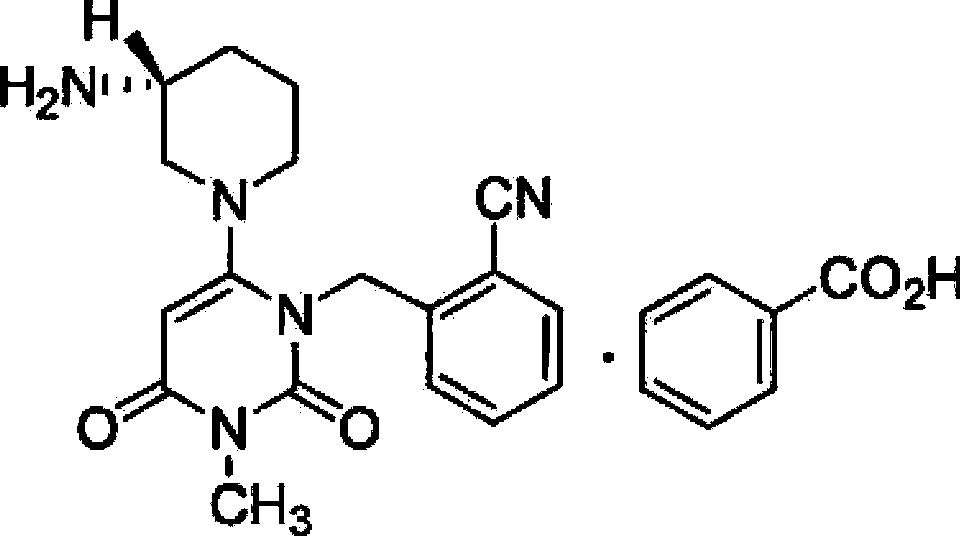
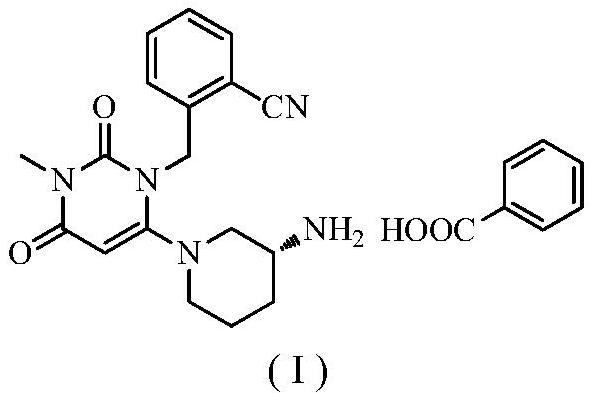
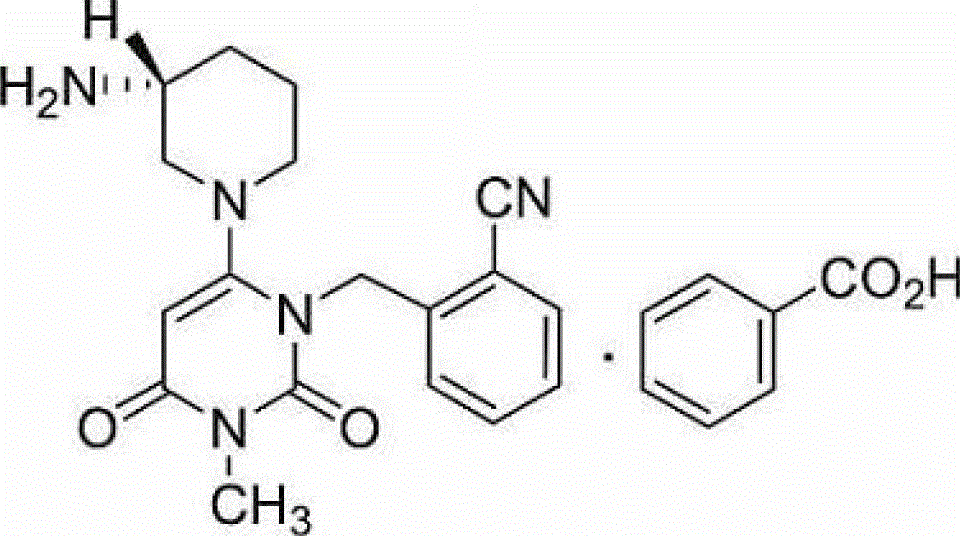
The benzoate salt form of alogliptin, a selective, orally bioavailable, pyrimidinedione-based inhibitor of dipeptidyl peptidase 4 (DPP-4), with hypoglycemic activity. In addition to its effect on glucose levels, alogliptin may inhibit inflammatory responses by preventing the toll-like receptor 4 (TLR-4)-mediated formation of proinflammatory cytokines.
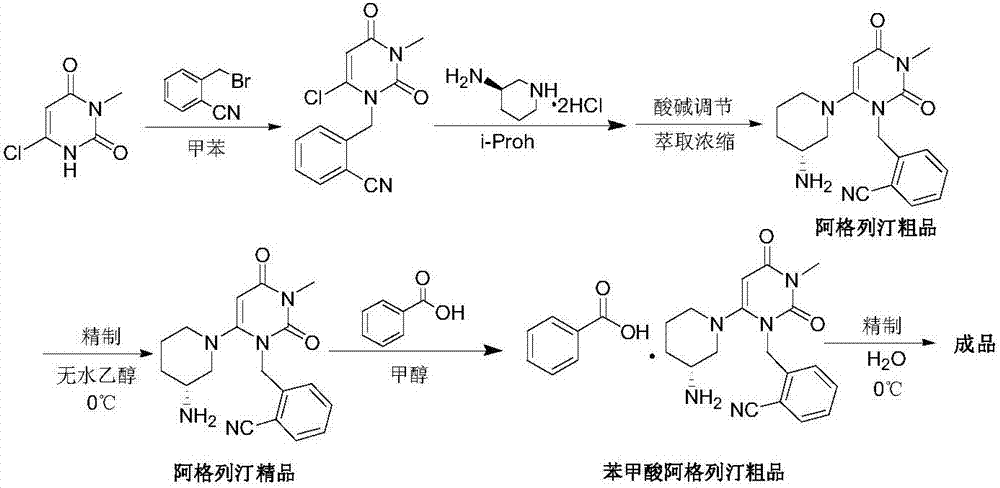
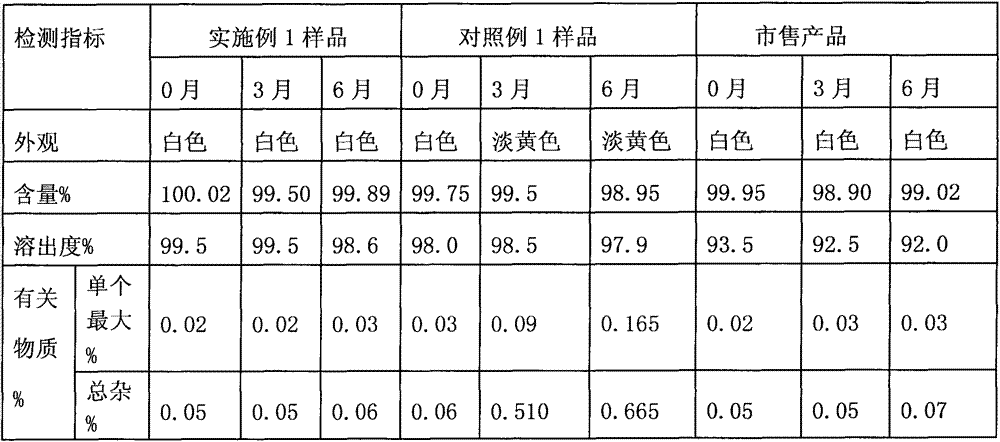
Preparation method of alogliptin benzoate
ActiveCN103193762ACarboxylic acid salt preparationBenzoic acidTert-Butyloxycarbonyl protecting group
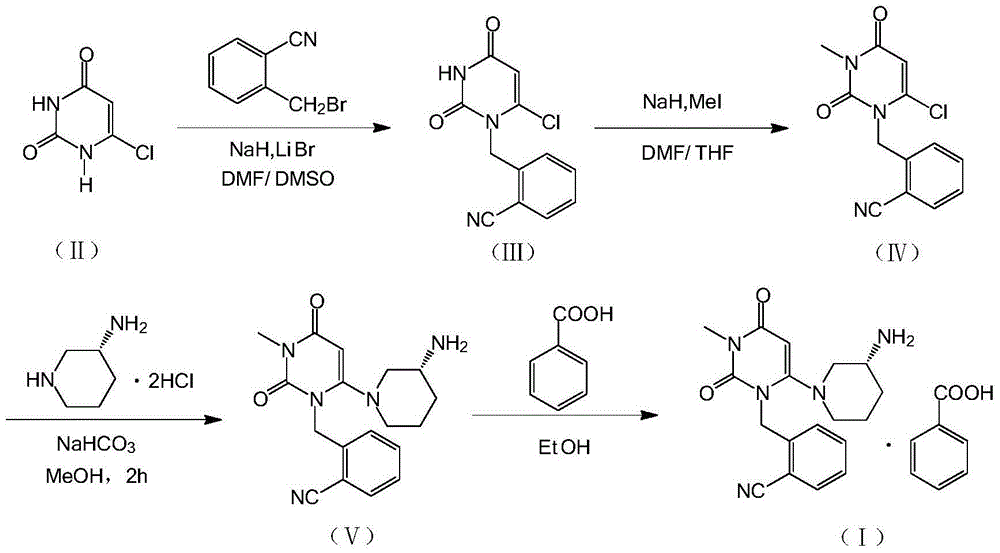
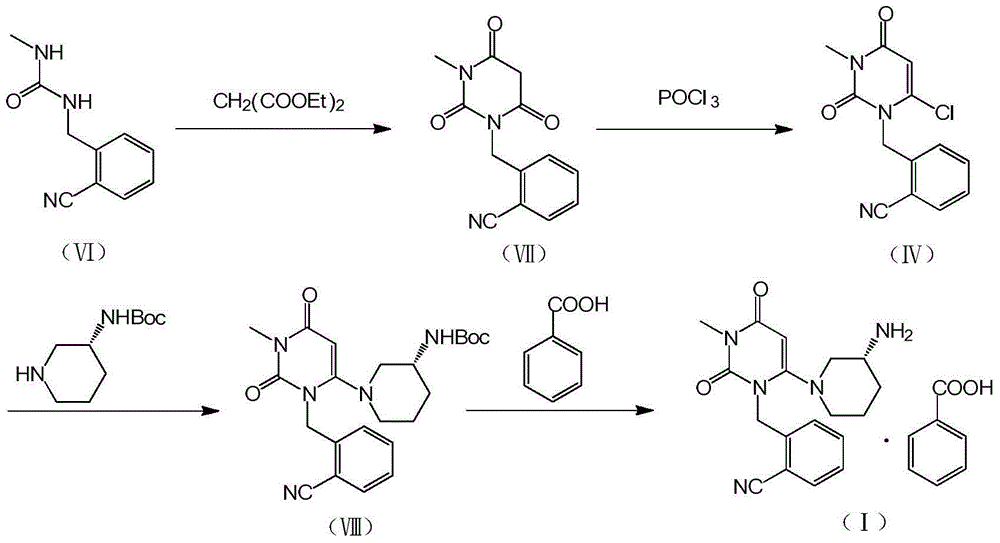
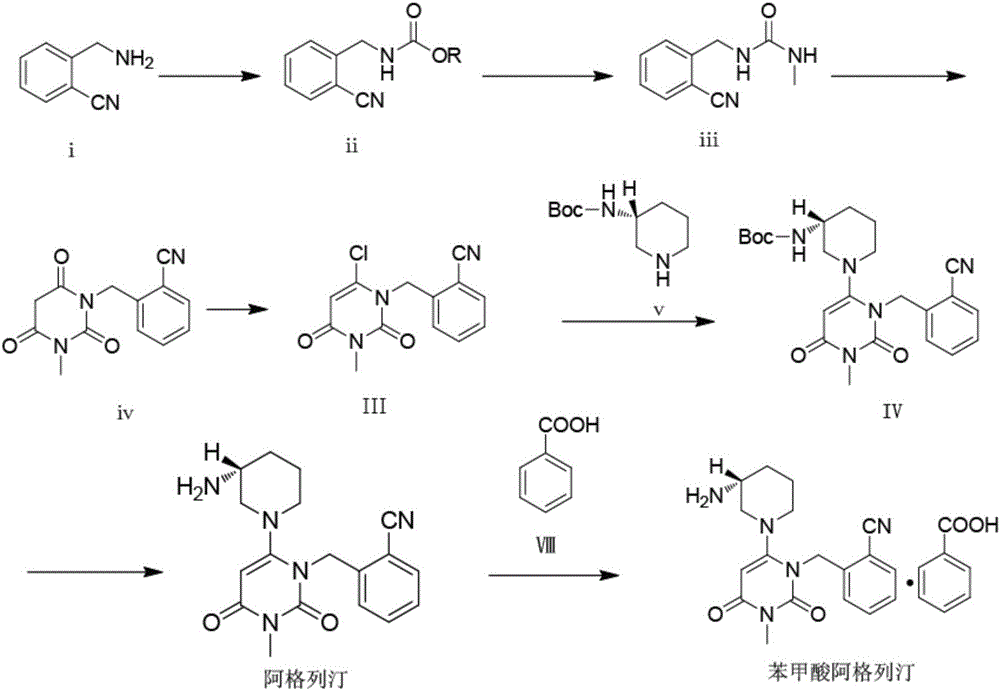
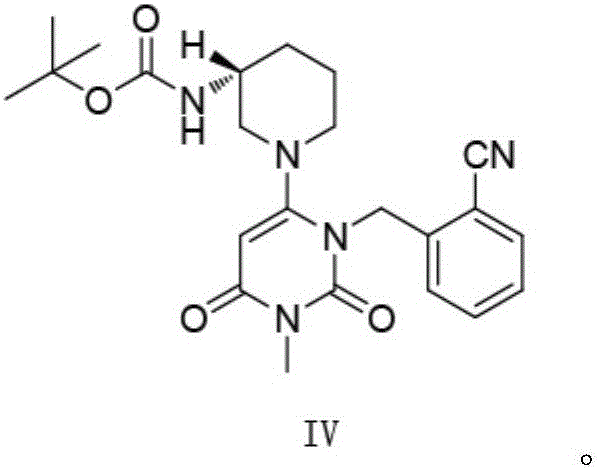
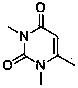
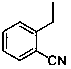
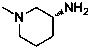
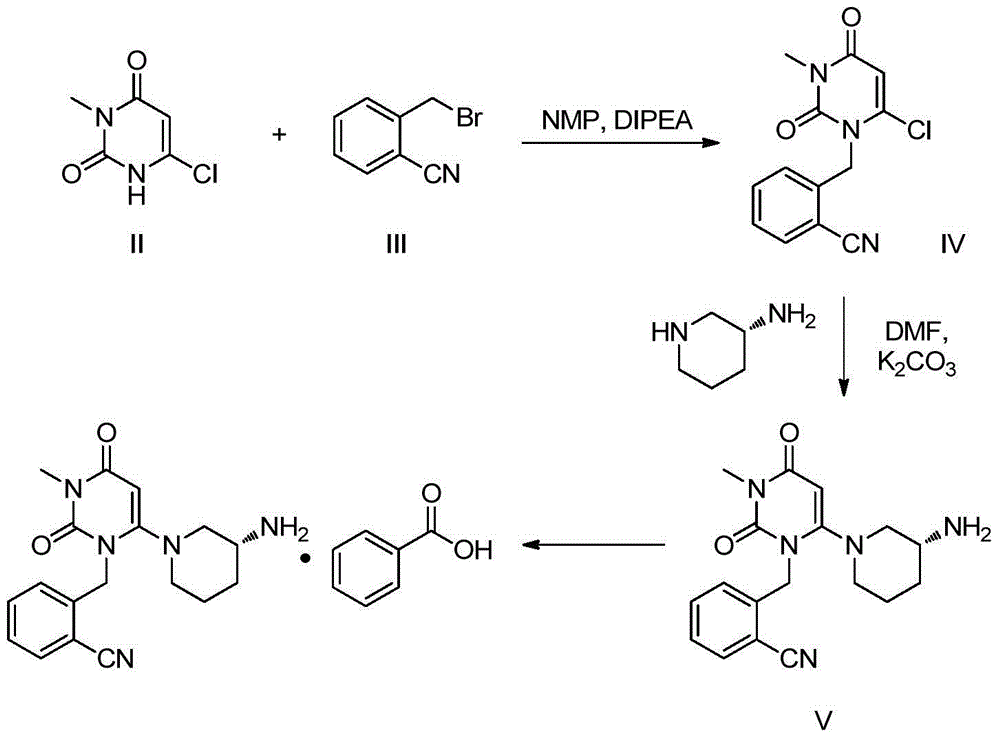
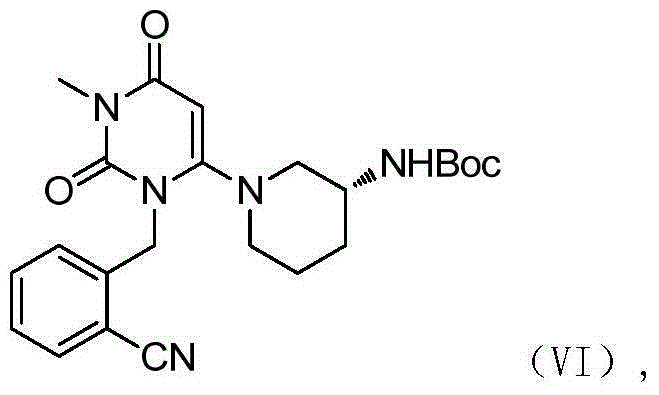
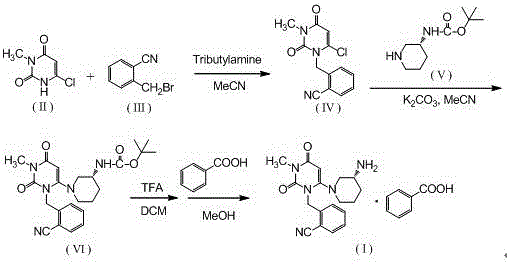
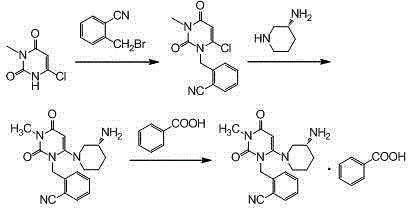
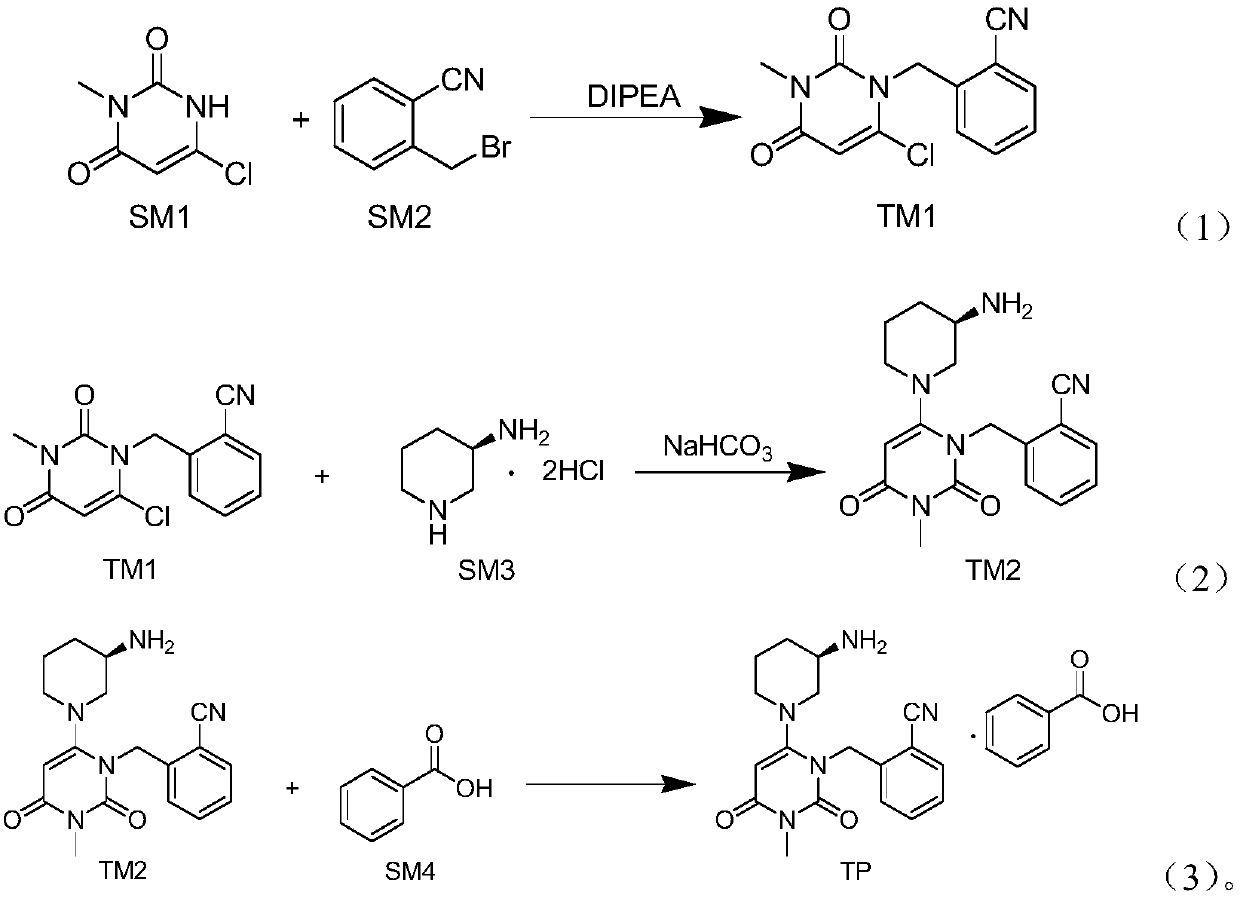
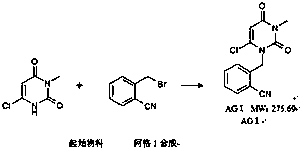
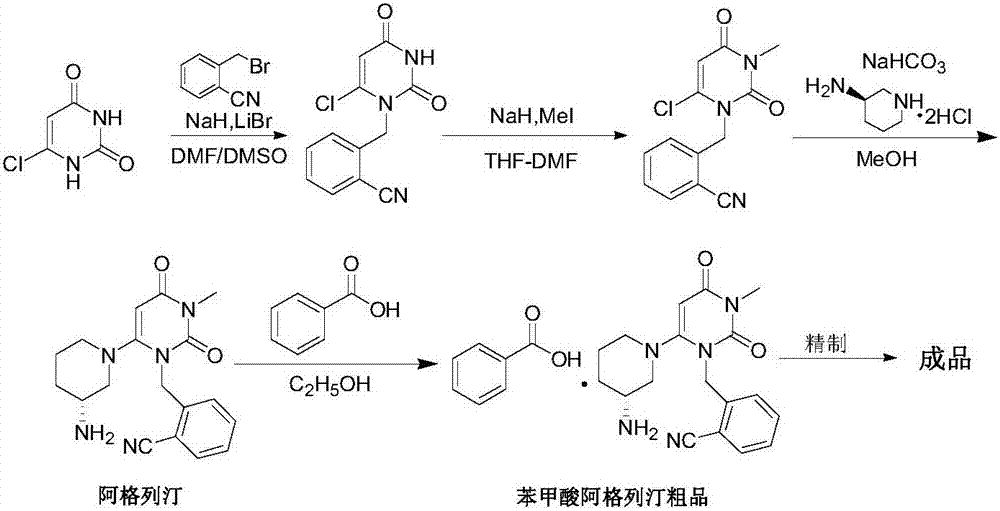
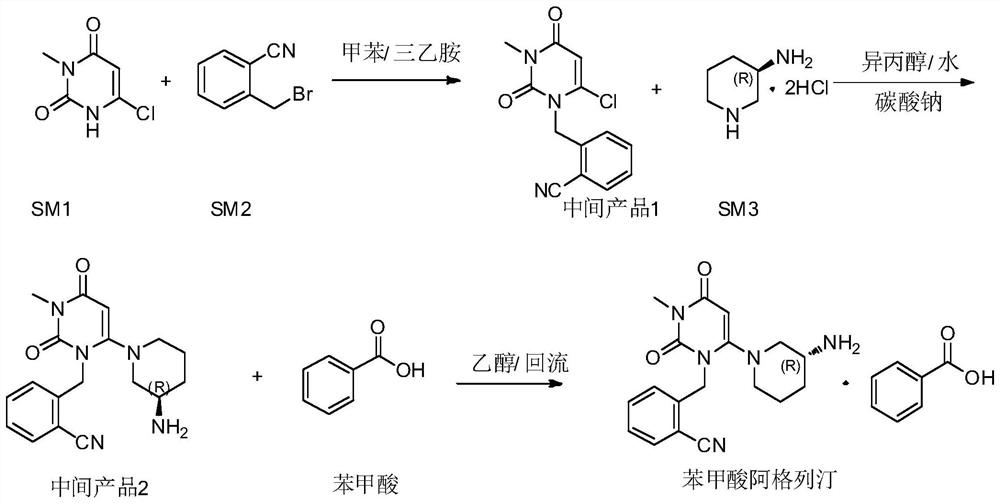
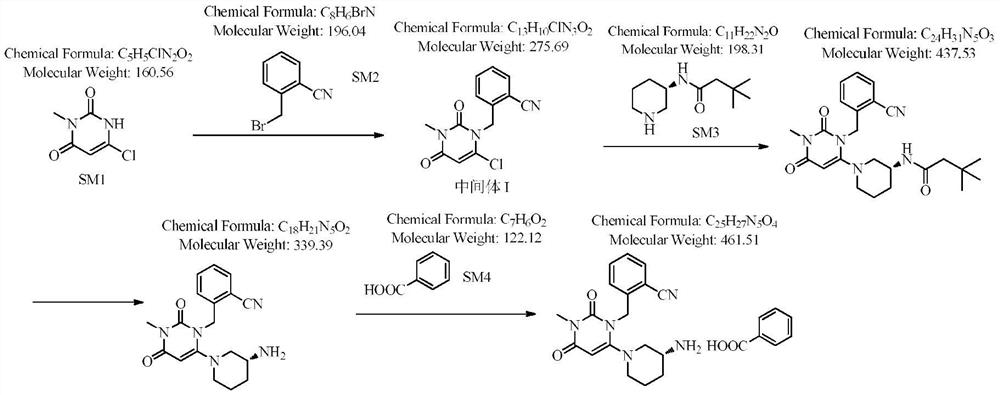
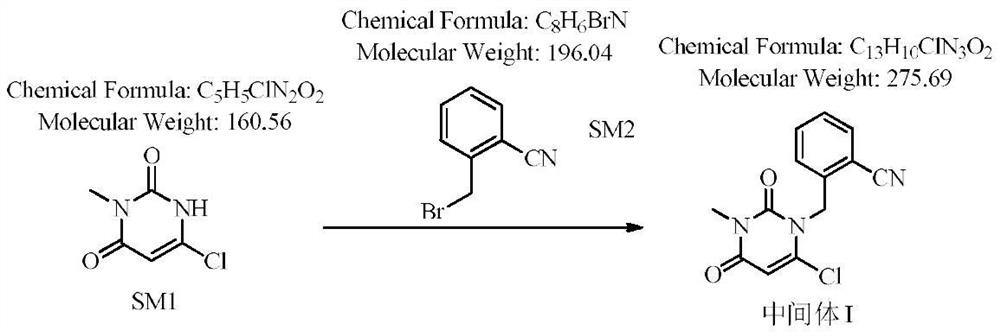
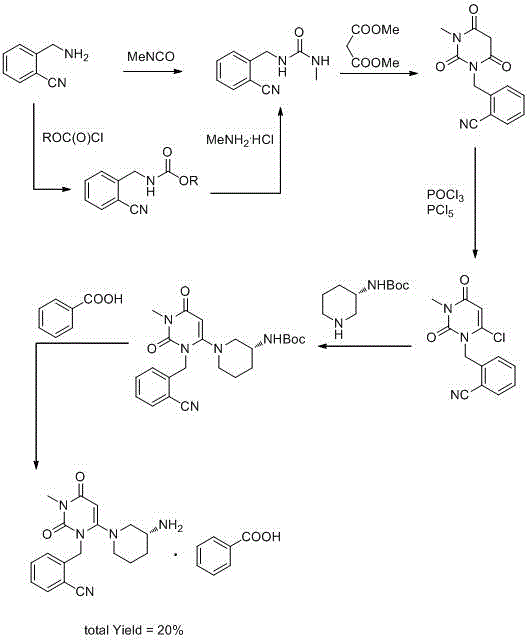
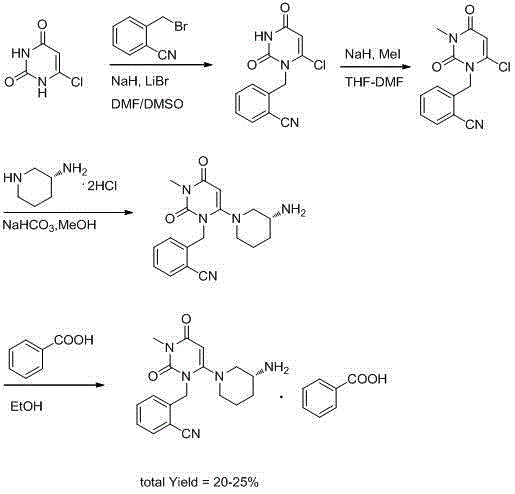
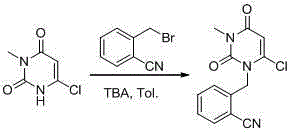
The invention relates to the field of preparation of medicaments and specifically relates to a preparation method of alogliptin benzoate. The preparation method comprises the following steps of: taking 6-chlorouracil as a starting raw material, reacting with o-cyanobenzyl bromide to obtain 2-((6-chloro-2, 4-dioxo-3, 4-dihydro-2H-pyrimidin-1-yl) methyl) benzonitrile, further performing methylation with iodomethane to obtain 2-((6-chloro-3-methyl-2, 4-dioxo-3, 4-dihydro-2H-pyrimidin-1-yl) methyl) benzonitrile, then reacting with (3R)-3-tert-butoxycarbonylamino-piperidine to obtain N-(3-(2-cyano-benzyl)-1-methyl-2, 6-dioxo-1, 2, 3, 6-tetrahydro-pyrimidin-4-yl) piperidine-(3R)-3-tert-butyl carbamate, performing deprotection by hydrogen chloride gas, and further forming a salt with benzoic acid to obtain the alogliptin benzoate. According to the preparation method of the alogliptin benzoate, disclosed by the invention, the raw materials which are low in cost and easy to purchase are selected, an alogliptin benzoate product is finally generated by reaction, and the overall manufacturing cost is low; and the temperature is strictly controlled in the steps, byproducts are few, the yield is high, and no toxicity is generated.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +2
Industrial production method of Alogliptin benzoate raw material medicine
InactiveCN104803976AShorten production timeReduce production energy consumptionOrganic chemistryBiotechnologyBenzoic acid
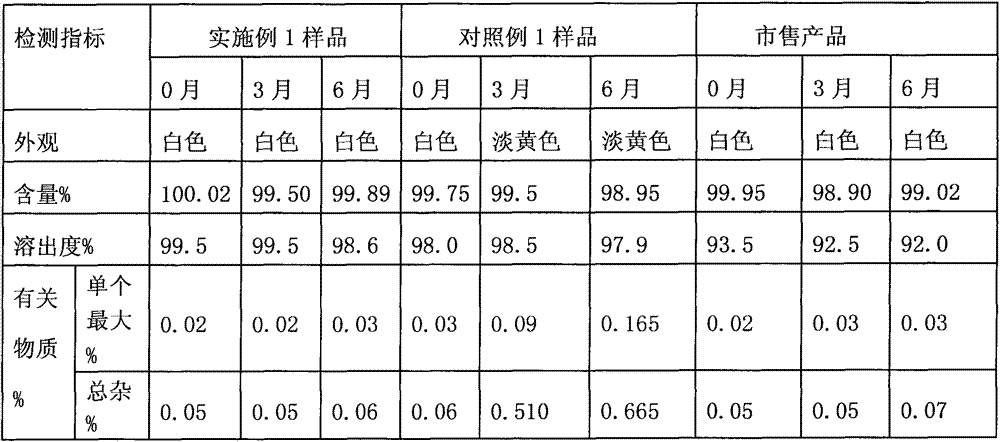
The invention discloses an industrial production method of Alogliptin benzoate raw material medicine. According to the method, ethanol / water mixed solvents are used as crystallization solvents; the ethanol / water mixed solvents and Alogliptin benzoate crude products are heated and flow back to a state that the solution is clear; the temperature is lowered, and crystal seeds are added; the gradient temperature reduction crystal separation is carried out; centrifugation and drying are carried out, and the Alogliptin benzoate raw material medicine is obtained. The adopted mixed solvents have low toxicity, and a better environment-friendly effect can be achieved. Compared with the method in the prior art, the industrial production method provided by the invention has the advantages that the solvent use type is reduced, the process is simple, the yield is high, and the production cost is reduced; through the method, the purity of the obtained Alogliptin benzoate finished product is at least 99.8 percent, the single impurity content is lower than or equal to 0.05 percent, the ethanol residue is low (lower than or equal to 0.1 percent) and is much lower than the limit of 0.5 percent of medical raw material medicine; impurities X generated by the reaction between phthalic acid and Alogliptin can be removed.
Owner:SUZHOU YABAO PHARMA R&D CO LTD
Novel method for preparing alogliptin benzoate
ActiveCN103819450ALow purityReduce manufacturing costCarboxylic acid salt preparationAlkyl transferEconomic benefits
The invention discloses a novel method for preparing alogliptin benzoate ((R)2-[6-[3(R)-aminopiperidin-1-yl]-3-methyl-2,4-dioxo-3,4-dihydropyrimidine-1(2H)-yl)methyl]benzonitrile benzoate). The method comprises the steps of alkylation, phase transfer catalysis reaction, catalytic hydrogenation, salt formation protection and the like. The process is novel in routes, short in the steps, high in reaction yield, and low in production costs; the method has larger implementing value and social economic benefit.
Owner:ZHEJIANG YONGNING PHARMA
Preparation method of alogliptin benzoate
ActiveCN103467445AEasy to buySuitable for industrial productionCarboxylic acid salt preparationMedicinal chemistryChemistry
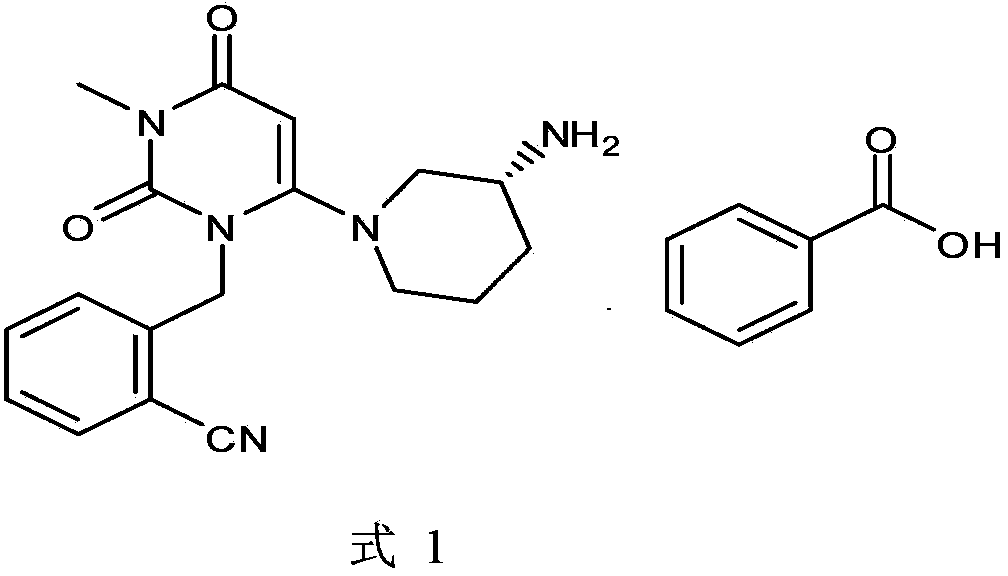
The invention discloses a preparation method of alogliptin benzoate (formula 1). The method is easily available in raw materials, mild in reaction conditions, simple in operation and applicable to large-scale industrial production.
Owner:SUZHOU LANXITE BIOTECH
Alogliptin benzoate preparation method
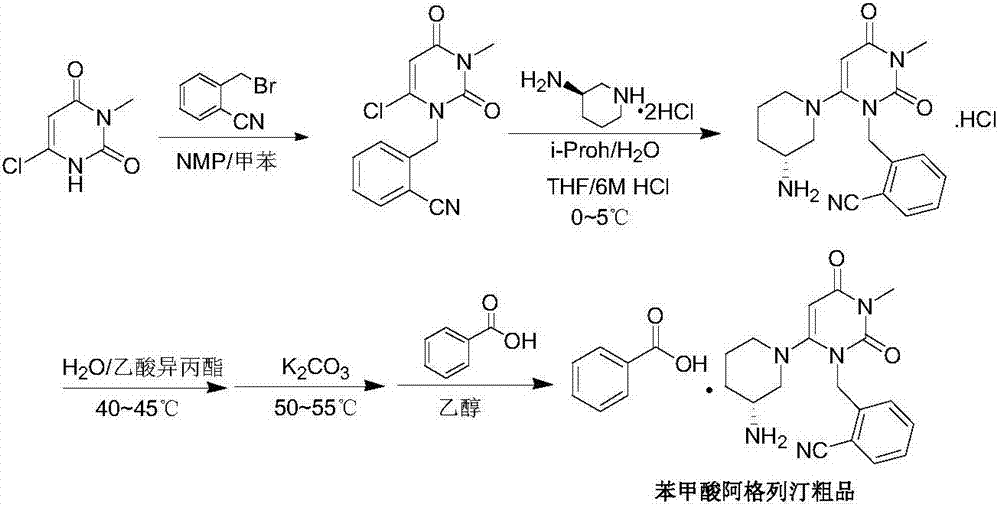
The present invention provides an alogliptin benzoate preparation method, which comprises treating an intermediate IV reaction solution, and specifically comprises: heating the intermediate IV reaction solution to a temperature of 50-80 DEG C, adding a diluted alcohol while hot, cooling to a temperature of 0-40 DEG C to make the crystal be crystallized, adding water after a lot of the crystals are crystallized, carrying out stirring washing, filtering, and drying to obtain the intermediate IV. According to the present invention, the intermediate IV is prepared by using the one-pot method, a certain amount of the diluted alcohol is added at the high temperature, the saturated solution is formed after the cooling, and the cooling is continuously performed to crystallize, such that the crystal caking problem caused by the direct water precipitation is avoided, the intermediate IV crystal is uniformly dispersed, sticking on the wall and the agglomeration do not exist, the sticking onto the stirring slurry does not exist, the operation is convenient, and the method is suitable for industrial production.
Owner:HEFEI LIFEON PHARMA
Method for measuring optical purity of R-alogliptin benzoate
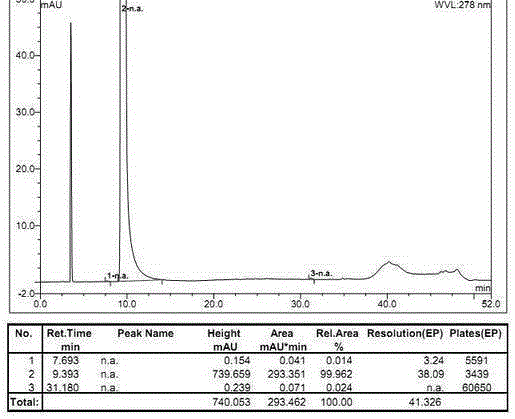
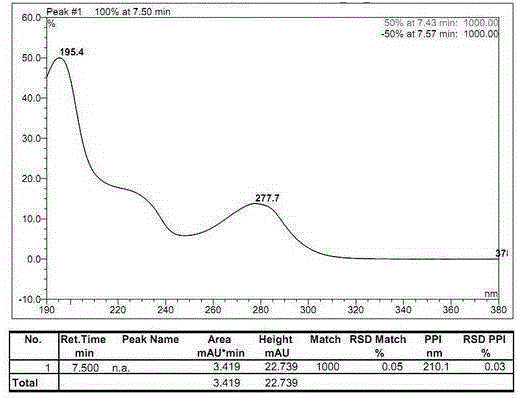
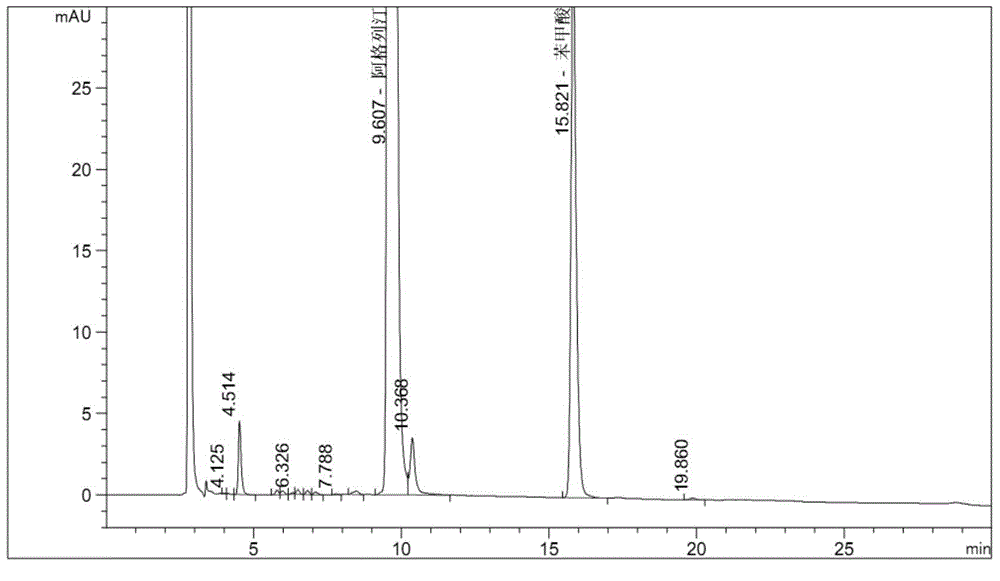
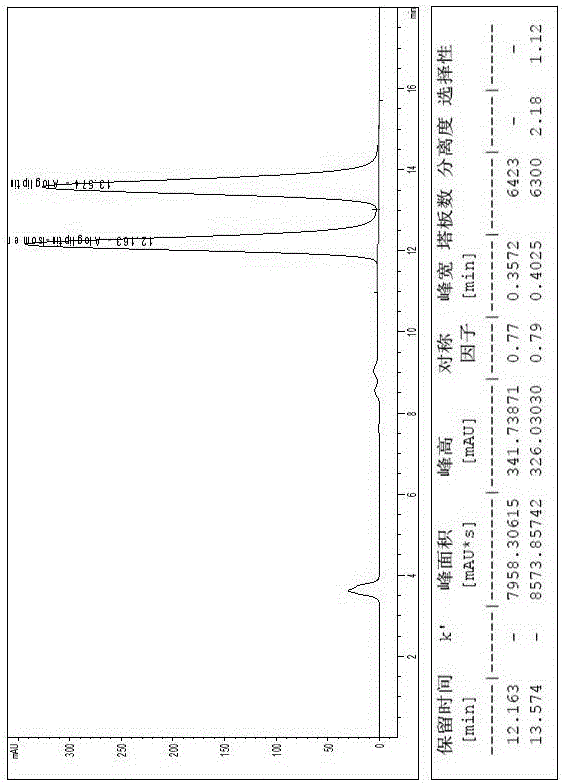
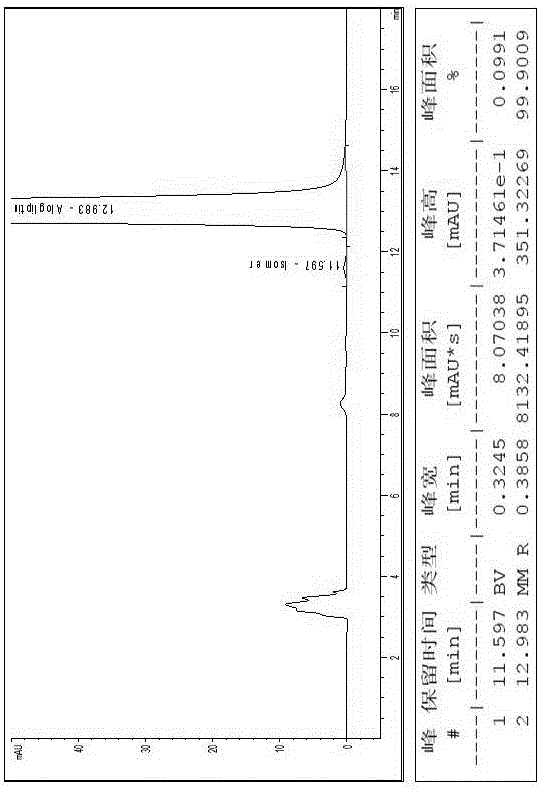
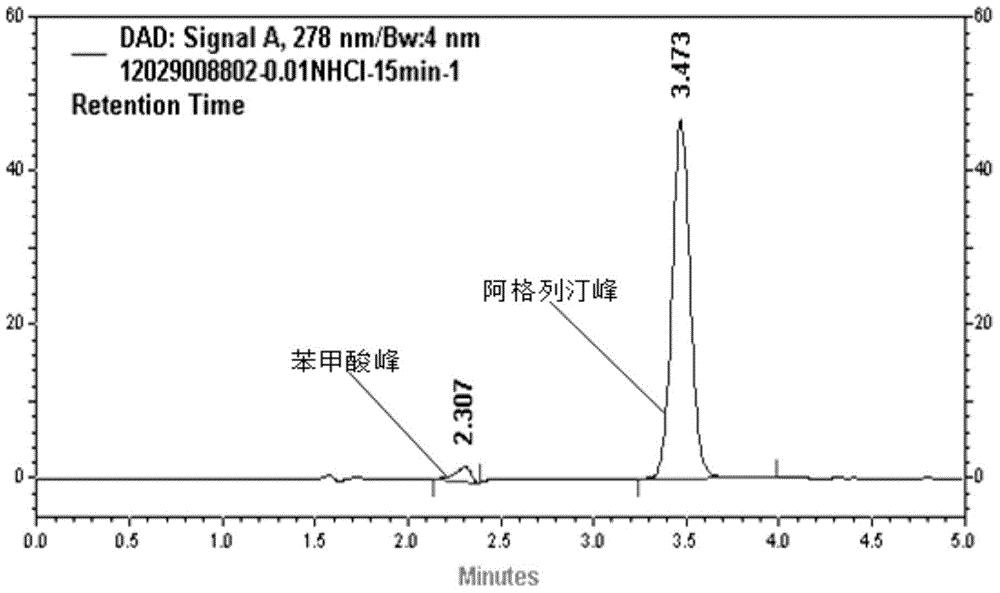
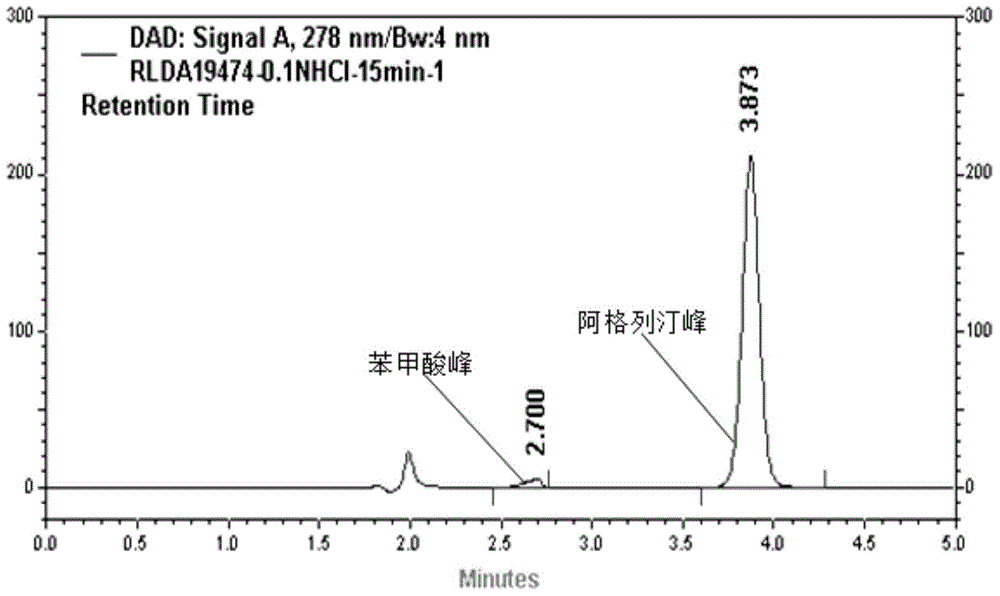
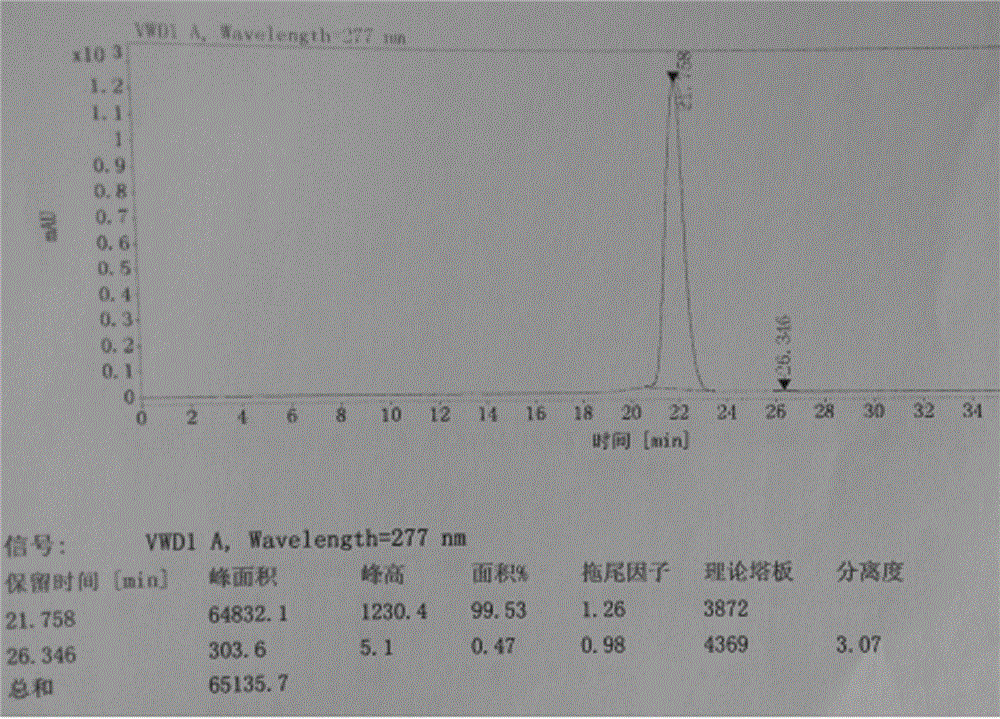
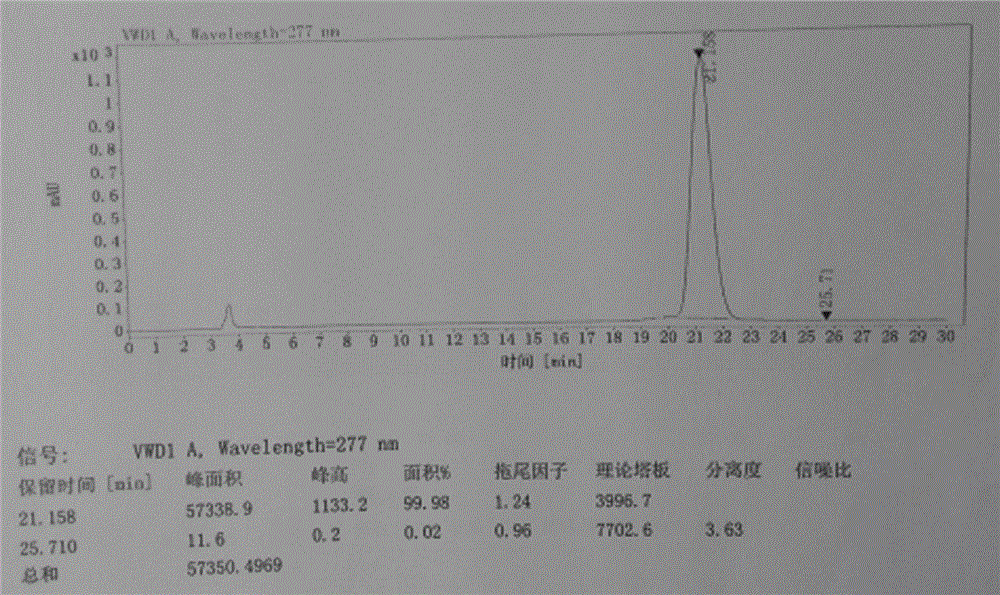
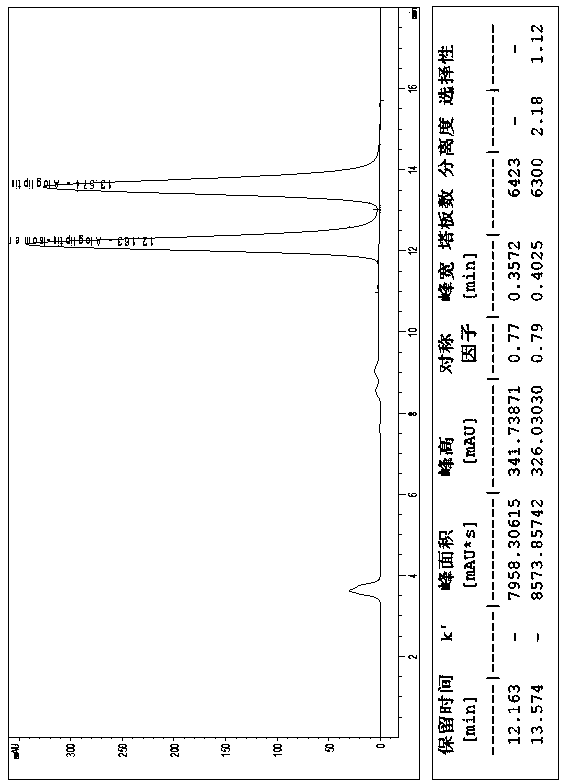
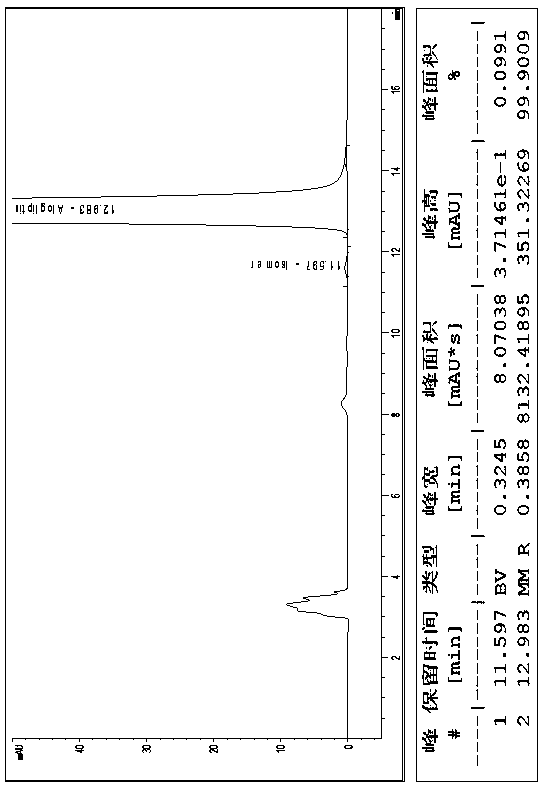
The invention relates to a method for measuring the optical purity of R-alogliptin benzoate. The method is characterized in that a chromatographic system is a high performance liquid chromatograph; a chromatographic column filler is amylose-tris(3,5-dimethylphenyl carbamate); the flow velocity is 0.8 ml / min; the column temperature is 25 DEG C; the measurement wavelength is 278 nm; the sample introduction amount is 20 [mu]l; the chromatographic mobile phases are n-hexane and an ethanol solution which contains 0.2% trifluoroacetic acid and 0.1% diethyl amine; the ratio of n-hexane to the ethanol solution is 80:20; sample introduction is conducted on an R,S-alogliptin benzoate reference solution and an S-alogliptin benzoate reference solution respectively for system suitability analysis at first, and then the optical purity of an R-alogliptin benzoate test solution is measured and the optical purity of an R-alogliptin benzoate sample is calculated according to an area normalization method.
Owner:DISHA PHARMA GRP +1
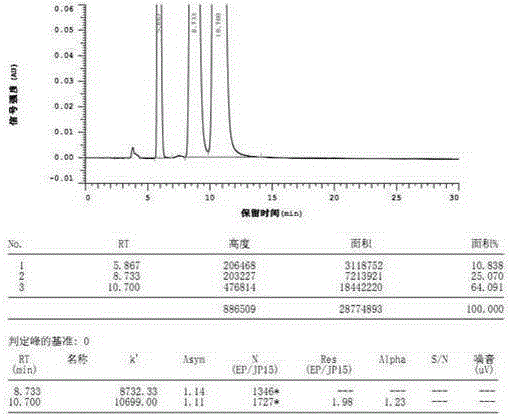
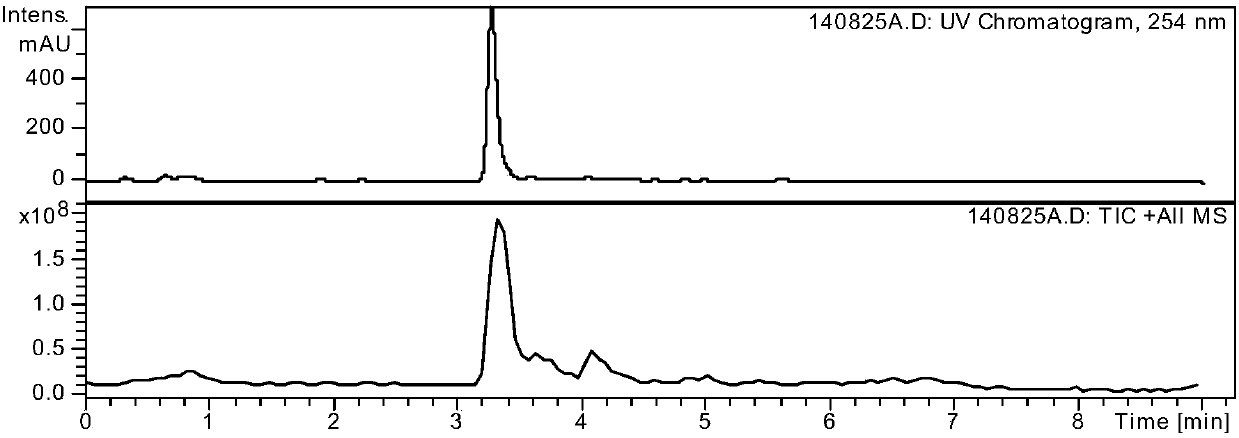
Method for separating and analyzing alogliptin benzoate and its related substance
ActiveCN105527348AEfficient separationAccurate separationComponent separationOrganic solventGradient elution
The invention provides a method for separating and analyzing alogliptin benzoate and its related substance. The method takes octyl bonded silica gel as a fixed phase and takes a mixed solvent of a mobile-phase A-buffer, a mobile-phase B-organic solvent as a mobile phase for gradient elution. The method can rapidly and efficiently separate alogliptin benzoate and its related substance under same chromatographic condition, and the detection method has the advantages of strong specialization, high precision, strong accuracy and convenient operation, and can effectively control the medicine quality.
Owner:WATERSTONE PHARMA WUHAN
Synthesis method of alogliptin benzoate
ActiveCN104193726AMild reaction conditionsEasy to operateCarboxylic acid salt preparationBenzoic acidBenzoyl bromide
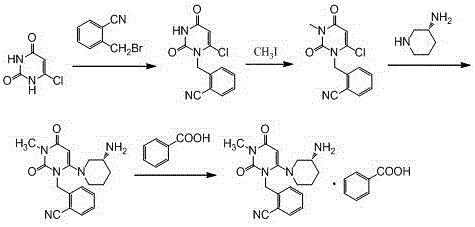
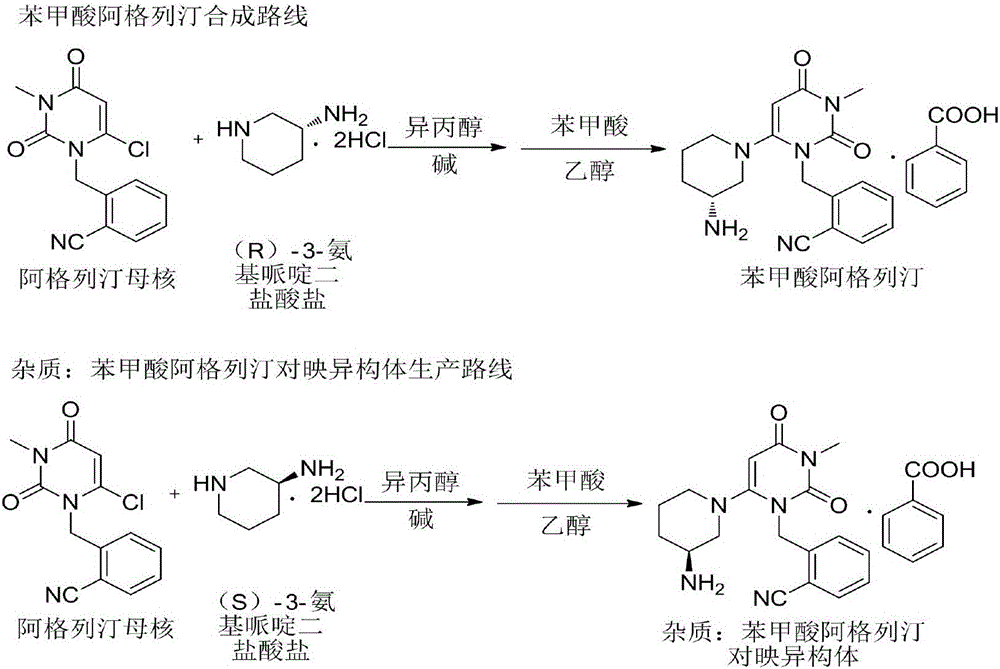
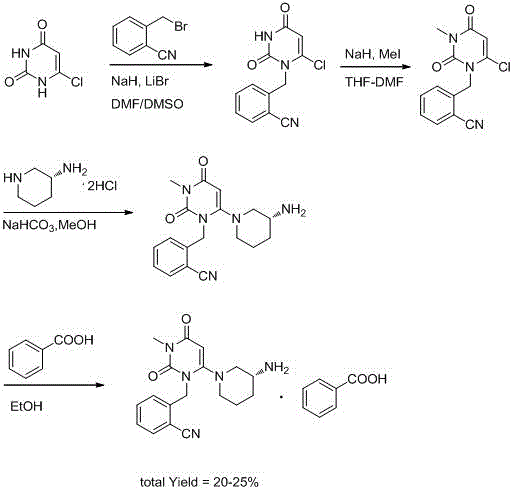
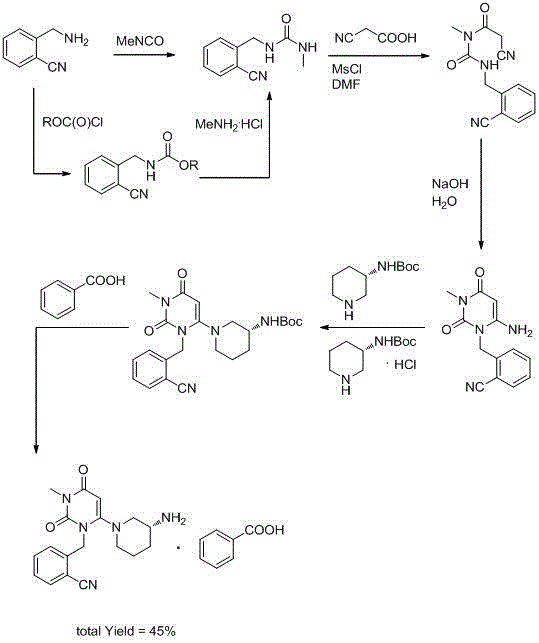
The invention discloses a synthesis method of alogliptin benzoate. The synthesis method comprises the following steps: putting 2-cyano benzyl bromide, 3-3metyl-6-chlorouracil and tri-n-butylamine in methylbenzene and stirring for reaction; cooling, adding water and stirring for crystallization; filtering and washing with water to obtain 2-(6-chlorine-3-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimdine-1-metyl)-benzonitrile; adding 2-(6-chlorine-3-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimdine-1-metyl)-benzonitrile, (R)-3-piperidinamine dihydrochloride and alkali into ethyl alcohol and stirring for reaction; purifying and salifying with benzoic acid to obtain alogliptin benzoate. The synthesis method disclosed by the invention is mild in condition, easy to control, non-toxic, environment-friendly, high in purity and high in yield.
Owner:JIANGSU DEYUAN PHARMA
Method for preparing DPP-IV inhibitor
InactiveCN104311535AEasy post-processingSimple and fast operationOrganic chemistryMethyl groupCarbonate
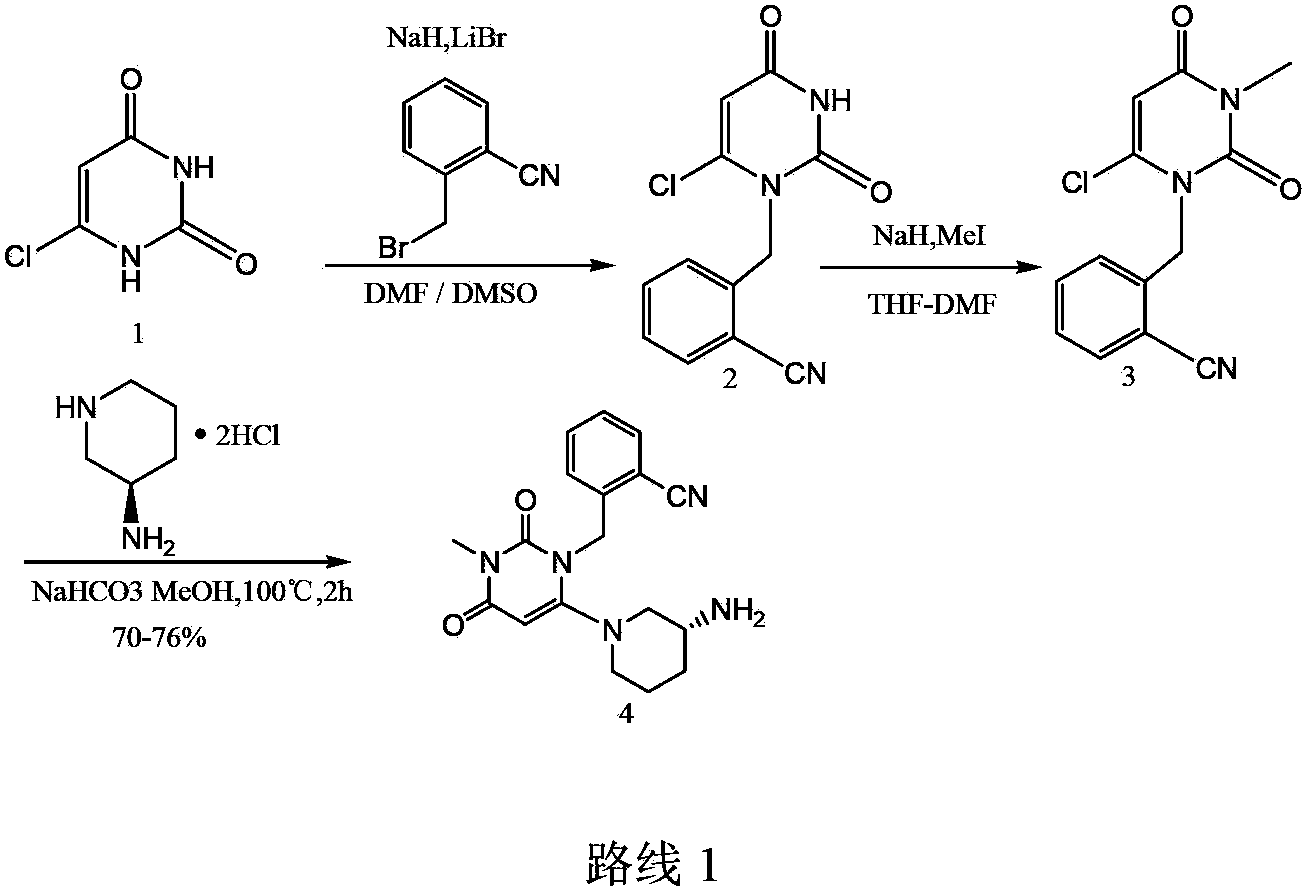
The invention relates to a method for preparing a DPP-IV inhibitor, and concretely relates to a method for preparing a modified alogliptin benzoate intermediate (R)-t-butyl{1-[3-(2-cyanbenzyl)-1-methyl-2,6-dioxo-1-1,2,3,6-tetrahydro-4-yl]piperidyl-3-yl}-t-butyl carbamate. The method comprises the following steps: 1, carrying out a nucleophilic substitution reaction on 6-chloro-3-methyluracil and 2-cyanobenzyl bromide in N,N-dimethyl formamide or N,N-dimethyl acetamide or an N,N-dimethyl formamide and N,N-dimethyl acetamide mixed solvent in the presence of an alkali metal carbonate; and 2, directly adding (R)-3-Boc-aminopiperidine and the alkali metal carbonate into the reaction solution obtained in step 1 for continuous reaction. The preparation method allows the reactions to be carried out in a same reactor and the intermediate to undergo a next step reaction without separation, so the method is simple to operate.
Owner:SUNSHINE LAKE PHARM CO LTD
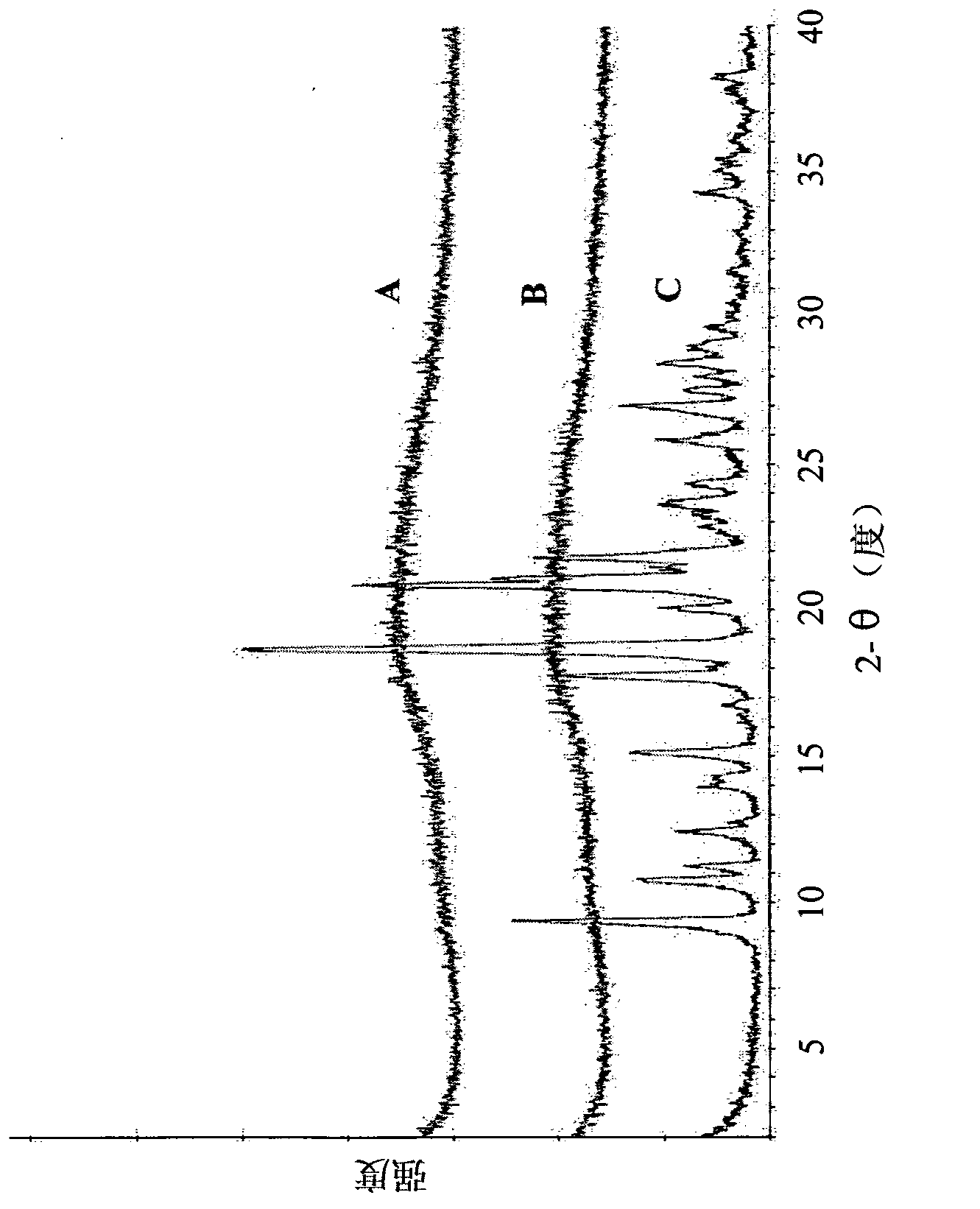
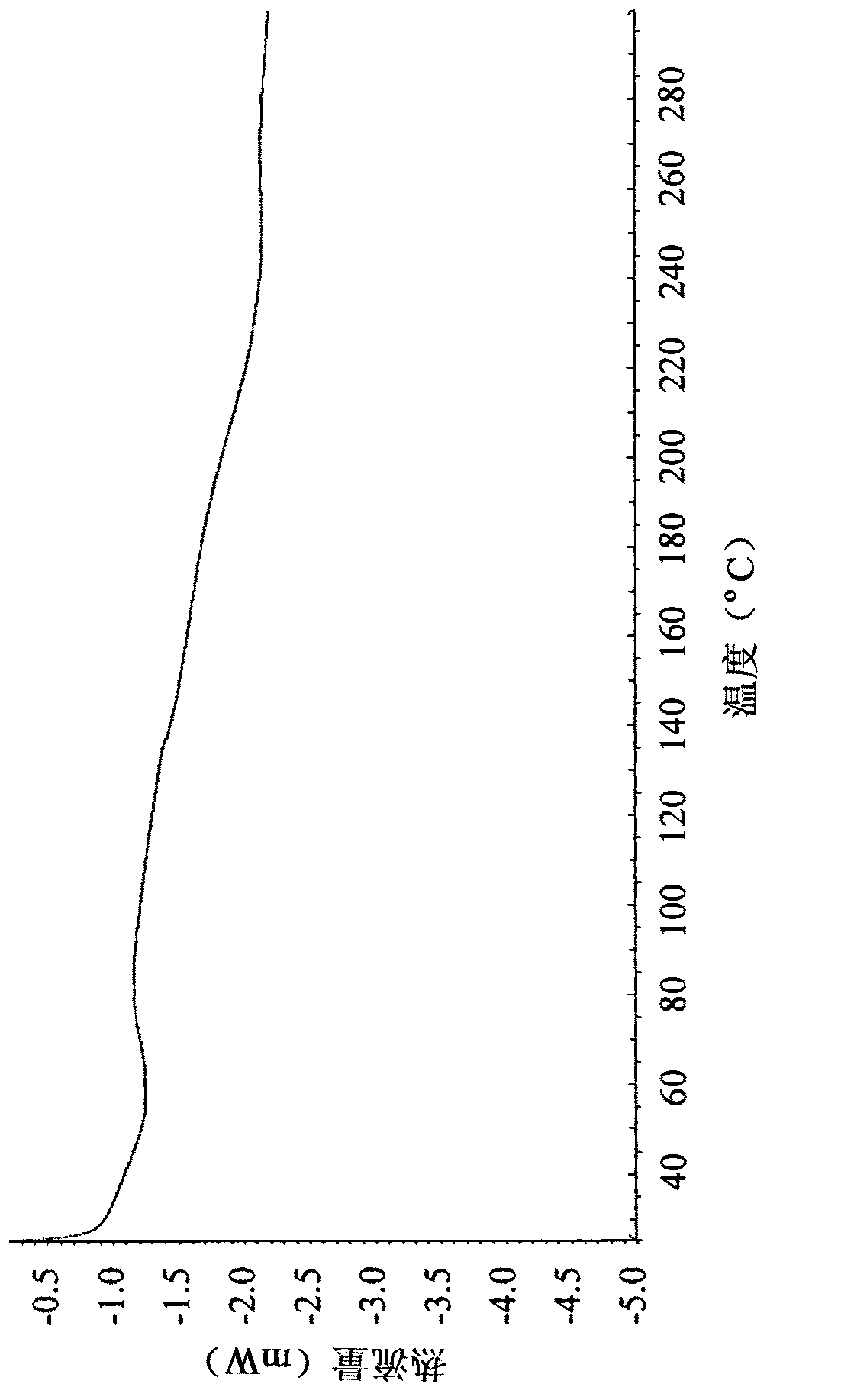
Polymorphs of alogliptin benzoate
The present invention provides new amorphous forms of alogliptin benzoate, pharmaceutical compositions comprising same, methods for their preparation and use thereof in treating conditions mediated by DPP-IV, in particular, type 2 diabetes.
Owner:MAPI PHARMA
Preparation method of alogliptin benzoate impurity
InactiveCN107556249AEasy to operateThe reaction conditions are mild and controllableOrganic chemistryBenzoic acidDiketone
The invention relates to a preparation method of alogliptin benzoate impurity and belongs to the technical field of pharmaceutical chemicals. The preparation method of alogliptin benzoate impurity provided by the invention comprises the following steps: (1) preparing TM1 2-((6-chloro-3-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)methyl)cyanophenyl by taking 6-chloro-3-methylpyrimidin-2,4(1H,3H)-diketone as a raw material which reacts with 2-bromomethyl cyanophenyl; (2) preparing alogliptin benzoate impurity BP1 through a reaction between the TM1 and monohydric alcohol in an alkaline condition, wherein the structural formula of the BP1 is shown in the description. The preparation method provided by the invention is convenient to operate, the reaction conditions are mild and controllable,the side reactions are reduced, and therefore, the target product alogliptin benzoate is easy to separate and purify and has high purity.
Owner:山东淄博新达制药有限公司
Method for separating Alogliptin benzoate and enantiomer thereof through high performance liquid chromatography
ActiveCN105929084AEffectively achieve separationEfficiently implement measurementsComponent separationCelluloseBenzoic acid
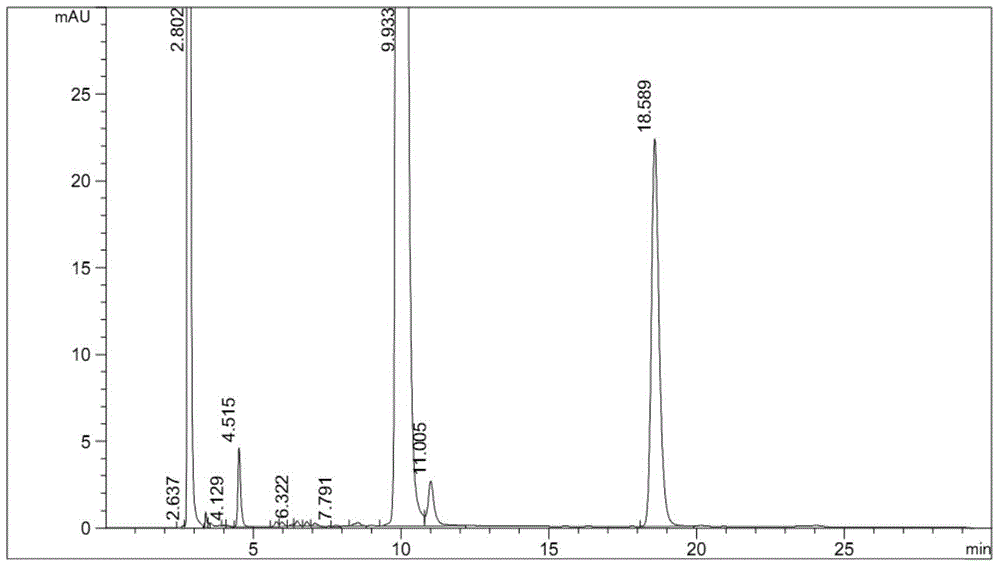
The invention discloses a method for separating Alogliptin benzoate and an enantiomer thereof through high performance liquid chromatography. The method comprises the following steps: dissolving an Alogliptin benzoate raw material medicine containing Alogliptin benzoate and the enantiomer thereof in a dilution solution until the concentration is 0.1-2mg / mL; and separating Alogliptin benzoate and the enantiomer thereof by using a high performance liquid chromatography system adopting silica with the surface being covalently bonded with cellulose-tri(3,5-dichlorophenylcarbamate) as a stationary phase and an n-hexane, ethyl acetate, anhydrous ethanol and triethylamine mixed solution as a mobile phase, wherein the dilution solution is one selected from or a mixture composed of two or more of acetonitrile, methanol and ethanol. The method effectively realizes separation and determination of Alogliptin benzoate and the enantiomer thereof, and guarantees the effectiveness and the safety of Alogliptin benzoate products.
Owner:JIANGSU DEYUAN PHARMA
Preparation and after-treatment method for high-purity alogliptin benzoate
InactiveCN106632242AEasy to operateLow costOrganic active ingredientsOrganic chemistryTemperature controlAfter treatment
The invention relates to optimization and improvement of a preparation and after-treatment method for alogliptin benzoate. In the Boc deprotection process, alkali extraction and acid precipitation methods are adopted, and impurities which do not contain basic groups are effectively removed on the basis of simplifying the process. Moreover, the solvent and temperature control is optimized in the salifying process, and the product quality and yield are improved.
Owner:HANGZHOU BIO SINCERITY PHARMA TECH CO LTD
Method for detecting dissolution rate of alogliptin benzoate tablets by using liquid chromatography
The invention provides a method for detecting dissolution rate of alogliptin benzoate tablets. The method comprises the following steps: by adopting a Welch Ultimate AQ-C18 5-micron chromatographic column of 4.6*150mm, taking a mixed solution of phosphate aqueous solution with the pH value of 5.5-6.5 and acetonitrile as a mobile phase, performing isocratic elution, and detecting the dissolution rate of the alogliptin benzoate tablets on the liquid chromatography system. The detection method provided by the invention is economic, rapid and high-efficiency, has high specificity and is suitable for detecting the dissolution rate of the alogliptin benzoate tablets under different dissolution medium conditions.
Owner:SUNSHINE LAKE PHARM CO LTD
Preparation method of alogliptin benzoate
InactiveCN107602535AEasy to operateThe reaction conditions are mild and controllableCarboxylic acid salt preparationChemical industryBenzoic acid
The invention relates to a preparation method of alogliptin benzoate and belongs to the technical field of the pharmaceutical and chemical industry. The preparation method comprises the following steps: (1) taking 6-chlorine-3-methylpyrimidine-2,4(1H,3H)-diketone as the raw material, reacting with 2-brooethyl cyanophenyl to prepare TM1; (2) enabling the TM1 and (R)-3-amino piperidine dihydrochloride to react under the alkaline condition by taking a polar non-proton substance and water as solvents to prepare (R)-2-((6-(3-aminopiperidine-1-base)-3-methyl-2,4-dioxo-3,4-dihydropyrimidine-1(2H)-base)-methyl) cyanophenyl(TM2); and (3) enabling the TM2 to react with the benzoic acid to prepare alogliptin benzoate. The preparation method is simple and convenient to operate, the occurrence of sideeffects is reduced, the reaction yield and the purity of a target product are increased, and the preparation method is favorable for industrial production.
Owner:山东淄博新达制药有限公司
Industrial production method of Alogliptin benzoate
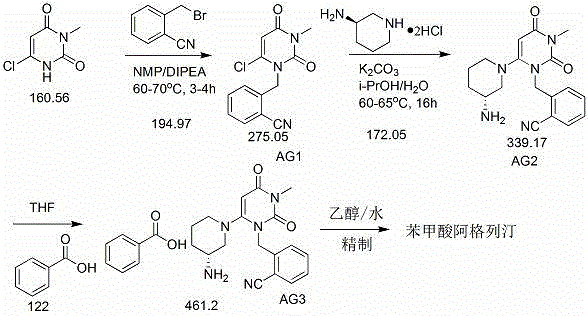
InactiveCN109232532AReduce pollutionAppropriate reaction conditionsCarboxylic acid salt preparationBenzoic acidAlogliptin
The invention relates to an industrial production method of Alogliptin benzoate, and belongs to the technical field of industrial pharmacy. The method comprises three steps of 1, preparing an Alogliptin intermediate AG I; 2, preparing an Alogliptin intermediate AG II; 3, preparing the Alogliptin benzoate. The industrial production method has the beneficial effects that the reaction conditions areproper; the operation is simple and convenient; the realization is easy; only the Alogliptin intermediate AG II is prepared; then, the AG II and benzoic acid are subjected to tetrahydrofuran synthesis; the Alogliptin benzoate can be obtained; the process is saved; the cost is reduced; used solvents have small environment pollution; the operation is safe; under the large-scale industrial productioncondition, the existing average yield is only about 30 percent; under the condition that the final yield reaches 19.32kg, the finial product yield reaches 94.77 percent; the total yield reaches 60.11percent; high quality and purity can be maintained; the production efficiency is greatly improved.
Owner:NANHAI PHARMA CHONGQING
Method for refining alogliptin benzoate
The invention relates to the field of pharmaceutical chemistry, and discloses a method for refining alogliptin benzoate. The method comprises the following steps: S1. A alogliptin benzoate crude product is added into an organic solvent for dissolving according to a proportion, wherein the proportion of alogliptin benzoate crude product to the organic solvent is 1g:2-10mL, the crude product is heated at 40-60 DEG C with stirring for dissolved clarification, wherein the organic solvent is selected from any one of acetone, tetrahydrofuran, alcohols and acetonitrile; S2. an aqueous solution of tartrate is added into liquid with dissolved clarification in the step S1, the aqueous solution of tartrate is added according to a proportion, wherein the proportion of the alogliptin benzoate crude product and the aqueous solution of tartrate is 1g:2-6mL, and heating is carried out at 40-60 DEG C with stirring for 1 hour; S2. cooling, crystallization and filtering are carried out in order to obtain solid without tartrate, the solid and benzoic acid form salt, crystallization and filtering are carried out, pressure reduction and drying are carried out for filter cakes, and a refined product of alogliptin benzoate is obtained. The refining method can reduce the content of enantiomer to 0.05% or below, refining yield reaches 89% or above, and safety and effectiveness of clinic application are guaranteed.
Owner:湖南千金湘江药业股份有限公司
Composition
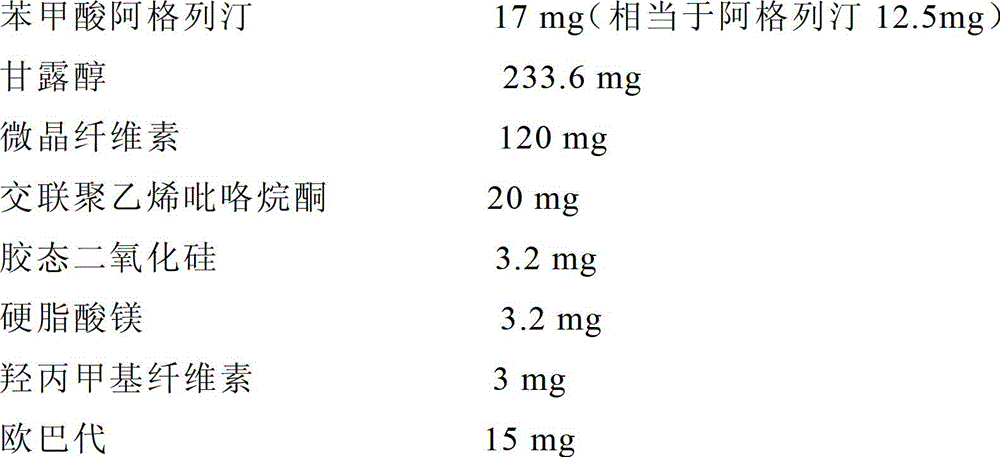
ActiveCN103610661ASolve the sticking problemSolve the problem of low dissolution rateOrganic active ingredientsMetabolism disorderCross-linkBenzoic acid
The invention relates to an alogliptin benzoate tablet composition. The alogliptin benzoate tablet composition is characterized by comprising 34 g of alogliptin benzoate, 2 to 12 g of silicon dioxide, 30 to 77.5 g of mannitol, 25 to 62.5 g of microcrystalline cellulose, 6 g of cross-linked sodium carboxymethyl cellulose, 4 g of hydroxypropylcellulose, 1.5 g of magnesium stearate and a suitable amount of opadry coating powder in every 1,000 tablets. Through reasonable compatibility and by the adoption of a direct pressing process, the production process is simplified, the production time is shortened, the production efficiency is improved and the product stability is improved compared with the conventional wet granulation process.
Owner:DISHA PHARMA GRP
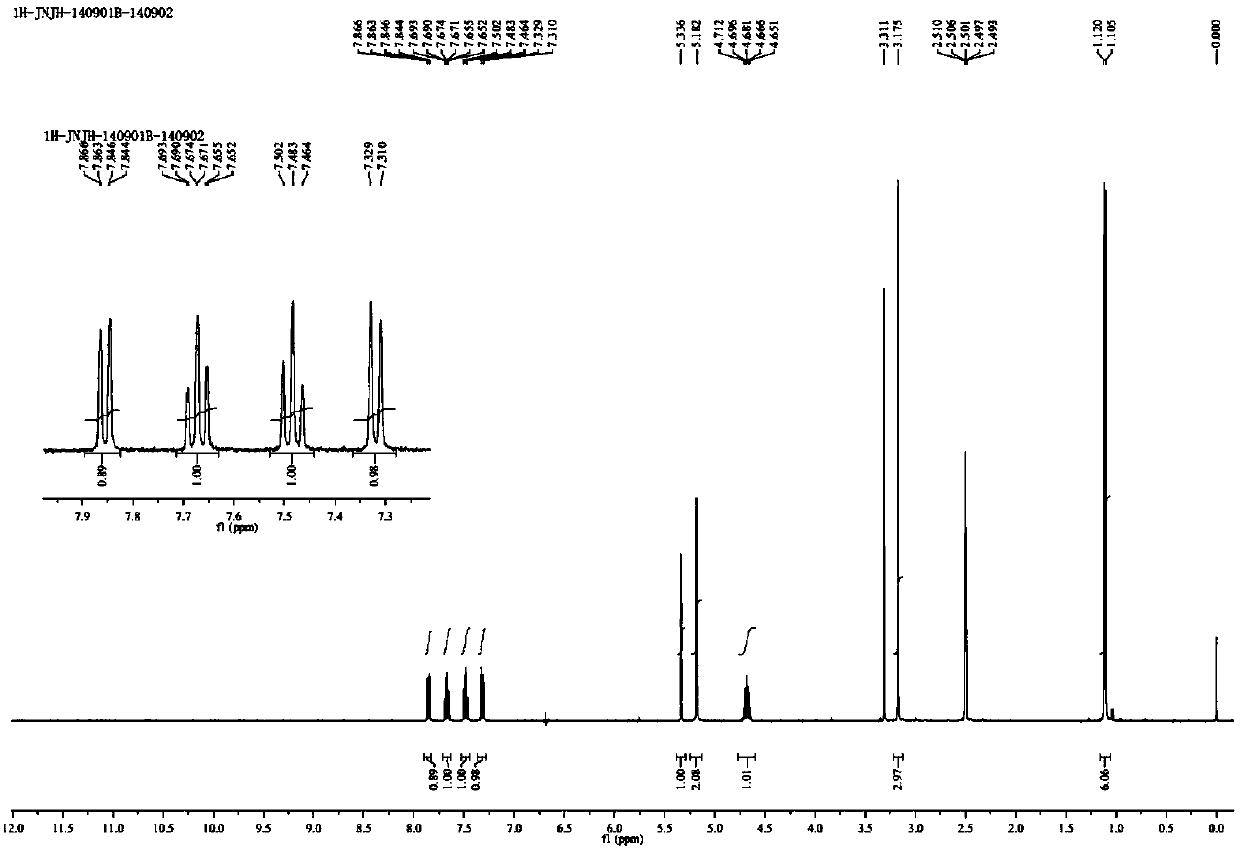
A preparing method for alogliptin benzoate
ActiveCN107540656AHigh purityHigh yieldCarboxylic acid salt preparationBenzonitrileDiabetes mellitus type II
The invention provides a preparing method for alogliptin benzoate, and particularly provides a method for preparing 2-[[6-[(3R)-3-aminopiperidin-1-yl]-3-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl]methyl]benzonitrile monobenzoate shown as a formula (I). The compound is a novel medicine treating diabetes mellitus type 2. The alogliptin benzoate (HPLC purity of which is greater than 99.95%) can be prepared in a high yield and high purity by the method, a process is simple and convenient to operate and the method is suitable for industrial production.
Owner:CHANGZHOU NO 4 PHARMA FACTORY
Alogliptin-metformin sustained-release tablet and preparation method thereof
InactiveCN105287581AExcellent sustained slow release effectSteady slow release effectOrganic active ingredientsMetabolism disorderSustained Release TabletImmediate release
The invention provides an alogliptin-metformin sustained-release tablet consisting of a metformin hydrochloride sustained-release portion and an alogliptin benzoate immediate-release portion. The alogliptin-metformin sustained-release tablet comprises the following ingredients by weight percentage as shown in the description. Through series of experiments, the alogliptin-metformin sustained-release tablet provides favorable conditions for rapid absorption of alogliptin benzoate in vivo, and metformin hydrochloride achieves more sustained and stable sustained-release effect. Compared with common preparations, the alogliptin-metformin sustained-release tablet has the advantages that the alogliptin-metformin sustained-release tablet is rapider and more stable in release and can enable a diabetes patient to keep stable blood sugar concentration for the whole day. The alogliptin-metformin sustained-release tablet is taken once per day conveniently and has the combination medication advantage and high clinical application value. A preparation method of the alogliptin-metformin sustained-release tablet is good in reproducibility and stability and suitable for industrial production.
Owner:SHANGHAI SUNTECH PHARMA
Alogliptin benzoate tablet and preparation method thereof
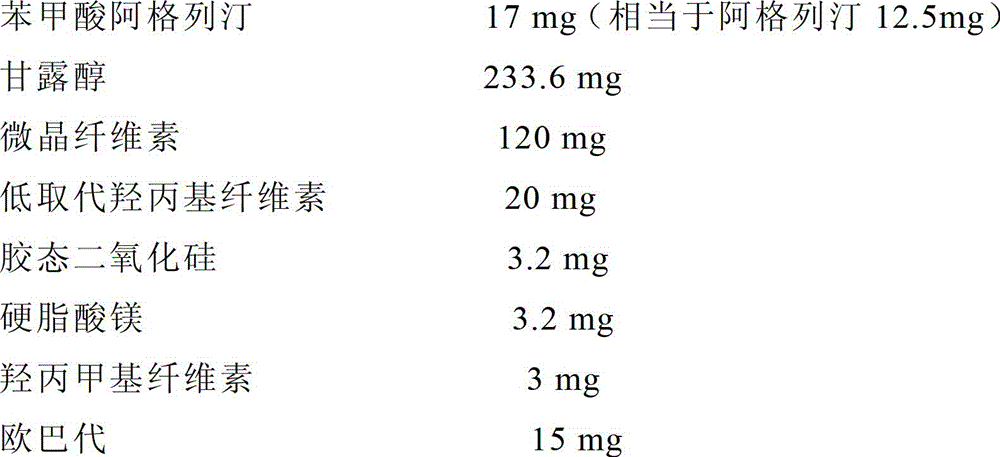
ActiveCN103877054ALow requirements for excipientsLow costOrganic active ingredientsMetabolism disorderBenzoic acidHigh humidity
The invention provides an alogliptin benzoate tablet and a preparation method thereof. The alogliptin benzoate tablet of the invention comprises alogliptin benzoate tablet, a disintegrating agent, a filler, an adhesive, a flow aid and a lubricant, wherein the disintegrating agent is selected from crosslinked polyvinylpyrrolidone or low-substituted hydroxy propyl cellulose. The method of the invention adopts wet granulation, is low in requirements for equipment and auxiliary materials, and reduces cost; the preparation steps are simple; the effective component of alogliptin benzoate in the tablet of the invention does not interact with the disintegrating agent; the tablet is good in dissolubility; in the preparation process, the adding mode of the disintegrating agent can be selected randomly; the alogliptin benzoate tablet of the invention has good stability, and can keep stable under conditions of high temperature and high humidity.
Owner:NEW FOUNDER HLDG DEV LLC +2
a composition
ActiveCN103610661BSolve the sticking problemSolve the problem of low dissolution rateOrganic active ingredientsMetabolism disorderMANNITOL/SORBITOLCroscarmellose sodium
The invention relates to a tablet composition containing alogliptin benzoate. It is characterized in that every 1000 tablets contain alogliptin benzoate 34g, silicon dioxide 2-12g, mannitol 30-77.5g, microcrystalline cellulose 25-62.5g, croscarmellose sodium 6g, hydroxyl Propylene cellulose 4g, magnesium stearate 1.5g, Opadry coating powder appropriate amount. Through reasonable compatibility, the present invention adopts the direct compression process, which simplifies the production process, shortens the production time, improves the production efficiency and improves the product stability compared with the traditional wet granulation process.
Owner:DISHA PHARMA GRP
Preparation method of alogliptin benzoate
ActiveCN107954978AMild reaction conditionsAvoid it happening againCarboxylic acid salt preparationBenzoic acidAlogliptin
The invention discloses a preparation method of alogliptin benzoate. The preparation method of alogliptin benzoate comprises the following steps of, firstly, reacting 3-methyl-6-chlorouracil with 2-cyanobenzyl bromide to obtain 2-(6-chloro-3-methyl-2, 4-dioxo-3, 4-dihydro-2H-pyrimidine-1-methyl)-benzonitrile; secondly, reacting 2-(6-chloro-3-methyl-2, 4-dioxo-3, 4-dihydro-2H-pyrimidine-1-methyl)-benzonitrile with (R)-3-aminoperidine dihydrochloride to obtain alogliptin; thirdly, salifying alogliptin with benzoic acid to obtain alogliptin benzoate. According to the preparation method of alogliptin benzoate, condensation reaction in the first step is implemented in dichloromethane solvent and under reflux conditions, thereby being mild in conditions and capable of achieving a yield higher than 90%; condensation reaction in the second step is implement in water or water-methylbenzene mixed solution to avoid production of disubstituted impurities and achieving high product purity.
Owner:CHANGZHOU SUNLIGHT PHARMA
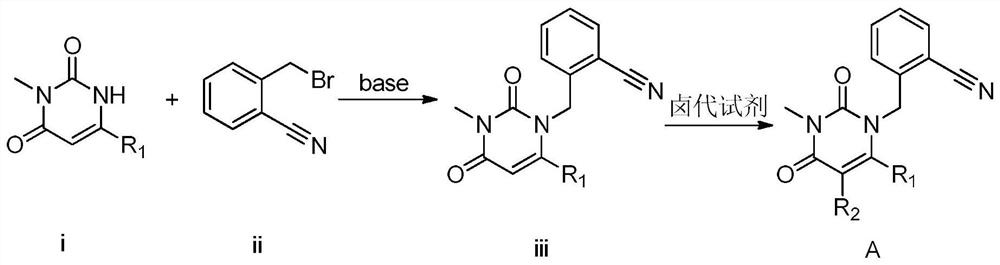
3, 4-dihydropyrimidine benzonitrile derivative and preparation method and application thereof
InactiveCN112480013AAchieve quality controlOrganic chemistryComponent separationBenzoic acidDihydropyrimidinuria
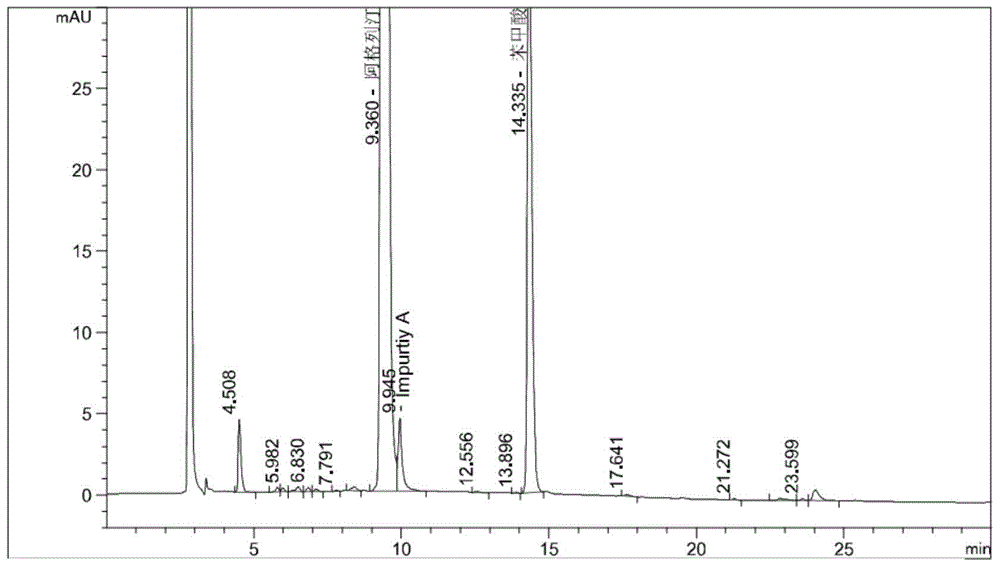
The invention provides a 3, 4-dihydropyrimidine benzonitrile derivative and a preparation method and application thereof. The invention discloses a structure of a specific impurity (RRT=1.22 impurity)generated in the process of preparing alogliptin benzoate by adopting the route of the original research patent for the first time. The series of impurities have a warning structure, and the contentof the impurities in the alogliptin benzoate finished product needs to be detected according to related limits of the genetically toxic impurities. The invention further provides a preparation and purification method of the high-purity compound shown in the formula (A) and a detection method of the content of the impurity compound in the alogliptin benzoate medicine, and therefore quality controlover the alogliptin benzoate medicine is achieved.
Owner:湖南千金湘江药业股份有限公司 +1
3, 4-dihydropyrimidine benzonitrile derivative as well as preparation method and application thereof
InactiveCN112480074AAchieve quality controlOrganic chemistryComponent separationBenzoic acidDihydropyrimidinuria
The invention provides a 3, 4-dihydropyrimidine benzonitrile derivative as well as a preparation method and application thereof. The invention discloses a structure of a specific impurity (RRT is an impurity of 1.22) generated in the process of preparing alogliptin benzoate by adopting the route of the original research patent for the first time. The series of impurities have a warning structure,and the content of the impurities in the alogliptin benzoate finished product needs to be detected according to related limits of the genetically toxic impurities. The invention further provides a preparation and purification method of the high-purity compound shown in the formula (A) and a detection method of the content of the impurity compound in the alogliptin benzoate medicine, and thereforequality control over the alogliptin benzoate medicine is achieved.
Owner:湖南千金湘江药业股份有限公司 +1
Novel preparation process of alogliptin benzoate
InactiveCN112759576ALow costHigh yieldOrganic compound preparationCarboxylic acid salt preparationBenzoic acidMethyl palmoxirate
The invention discloses a novel preparation process of alogliptin benzoate. The method comprises the steps: reacting 3-methyl-6-chlorouracil serving as an initial raw material with 2-cyanobenzyl bromide under an alkaline condition by taking toluene as a solvent to obtain 2-[(6-chloro-3,4-dihydro-3-methyl-2,4-dioxo-1(2H)-pyrimidinyl)methyl]benzonitrile, then reacting the solvent system with (R)-3-Boc-aminopiperidine under an alkaline condition, dissociating a protecting group by using acid to obtain alogliptin, and carrying out salifying reaction on the alogliptin and benzoic acid to finally prepare the alogliptin benzoate which is an anti-type 2 diabetes medicine. The preparation method adopts a one-pot method, has the advantages of low raw material cost, high yield, reduction of post-treatment operation of each chemical reaction in multi-step reaction, great shortening of the production period, few impurities generated in the reaction, high product quality, relative reduction of the use amount of chemical reagents, relatively environmental protection and the like, and is beneficial to industrial production.
Owner:山东永丞制药有限公司
A kind of synthetic method of alogliptin benzoate
ActiveCN104193726BMild reaction conditionsEasy to operateCarboxylic acid salt preparationBenzoic acidBenzoyl bromide
The invention discloses a synthesis method of alogliptin benzoate. The synthesis method comprises the following steps: putting 2-cyano benzyl bromide, 3-3metyl-6-chlorouracil and tri-n-butylamine in methylbenzene and stirring for reaction; cooling, adding water and stirring for crystallization; filtering and washing with water to obtain 2-(6-chlorine-3-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimdine-1-metyl)-benzonitrile; adding 2-(6-chlorine-3-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimdine-1-metyl)-benzonitrile, (R)-3-piperidinamine dihydrochloride and alkali into ethyl alcohol and stirring for reaction; purifying and salifying with benzoic acid to obtain alogliptin benzoate. The synthesis method disclosed by the invention is mild in condition, easy to control, non-toxic, environment-friendly, high in purity and high in yield.
Owner:JIANGSU DEYUAN PHARMA
Method for separating alogliptin benzoate and its enantiomers by high performance liquid chromatography
The invention discloses a method for separating Alogliptin benzoate and an enantiomer thereof through high performance liquid chromatography. The method comprises the following steps: dissolving an Alogliptin benzoate raw material medicine containing Alogliptin benzoate and the enantiomer thereof in a dilution solution until the concentration is 0.1-2mg / mL; and separating Alogliptin benzoate and the enantiomer thereof by using a high performance liquid chromatography system adopting silica with the surface being covalently bonded with cellulose-tri(3,5-dichlorophenylcarbamate) as a stationary phase and an n-hexane, ethyl acetate, anhydrous ethanol and triethylamine mixed solution as a mobile phase, wherein the dilution solution is one selected from or a mixture composed of two or more of acetonitrile, methanol and ethanol. The method effectively realizes separation and determination of Alogliptin benzoate and the enantiomer thereof, and guarantees the effectiveness and the safety of Alogliptin benzoate products.
Owner:JIANGSU DEYUAN PHARMA
A kind of alogliptin benzoate tablet and preparation method thereof
ActiveCN103877054BLow requirements for excipientsLow costOrganic active ingredientsMetabolism disorderBenzoic acidHigh humidity
The invention provides an alogliptin benzoate tablet and a preparation method thereof. The alogliptin benzoate tablet of the invention comprises alogliptin benzoate tablet, a disintegrating agent, a filler, an adhesive, a flow aid and a lubricant, wherein the disintegrating agent is selected from crosslinked polyvinylpyrrolidone or low-substituted hydroxy propyl cellulose. The method of the invention adopts wet granulation, is low in requirements for equipment and auxiliary materials, and reduces cost; the preparation steps are simple; the effective component of alogliptin benzoate in the tablet of the invention does not interact with the disintegrating agent; the tablet is good in dissolubility; in the preparation process, the adding mode of the disintegrating agent can be selected randomly; the alogliptin benzoate tablet of the invention has good stability, and can keep stable under conditions of high temperature and high humidity.
Owner:NEW FOUNDER HLDG DEV LLC +2
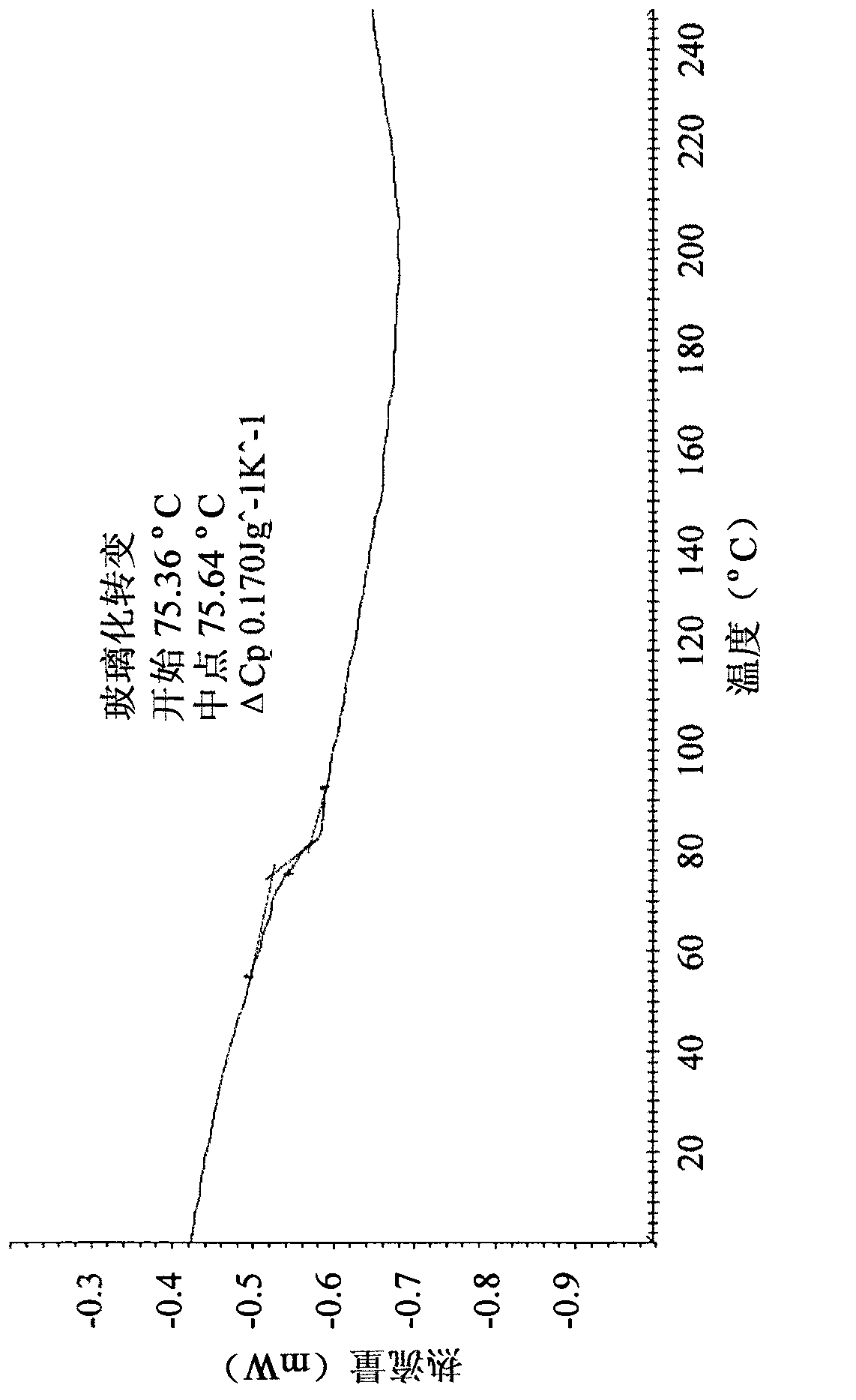
A kind of preparation method of alogliptin benzoate polymorph crystal
ActiveCN104151291BReduce pollutionMild reaction conditionsOrganic chemistry methodsBenzoic acidImpurity
The invention relates to a preparation method for a benzoic acid alogliptin polycrystalline type crystal and belongs to the technical field of medicinal chemistry. The preparation method comprises the following steps: methyl alcohol is added into a reaction still and stirred, benzoic acid alogliptin crude product obtained after synthesis is added, heating reflux is carried out for 15-30 minutes, the benzoic acid alogliptin crude product is filter-pressed into a refining-drying-packing crystallization kettle and stirred, methyl tertiary butyl ether is pressed into the crystallization kettle, the mixture is subjected to natural cooling to the room temperature, stirring and crystallization for 3-4 hours and centrifugation to obtain a solid, and the solid is washed with methyl alcohol and dried at 45-55 DEG C through blasting to obtain the benzoic acid alogliptin polycrystalline type crystal. The obtained benzoic acid alogliptin polycrystalline type crystal is low in impurity content and higher in medicine quality.
Owner:JIANGSU DEYUAN PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com