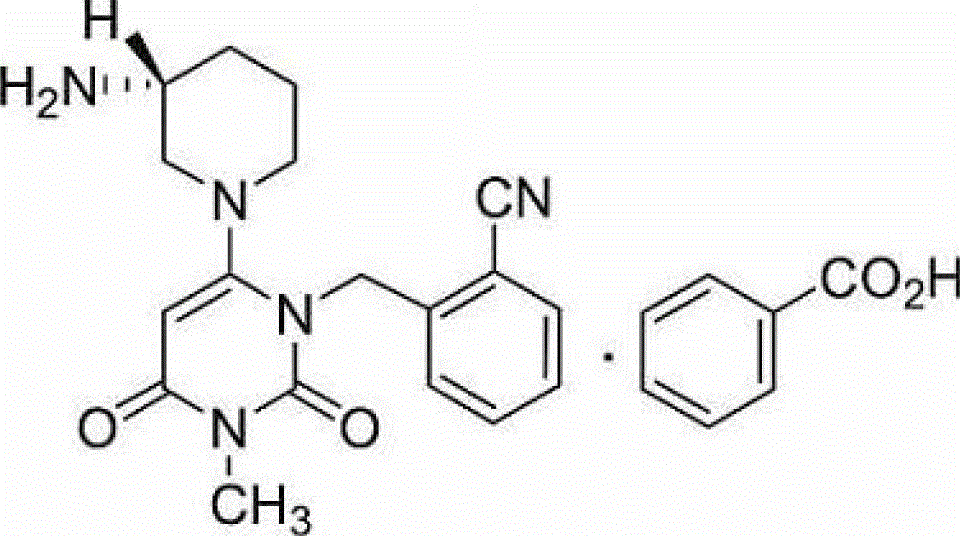
A kind of alogliptin benzoate tablet and preparation method thereof
A technology for alogliptin tablets and benzoic acid, which is applied in the field of alogliptin benzoate tablets and preparation thereof, can solve the problems of complicated steps, high equipment requirements and high cost, and achieves simple preparation steps, good stability, good dissolution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0073] The method for preparing alogliptin benzoate tablet of the present invention can also be such method, and it comprises the following steps:
[0074] i) Mix alogliptin benzoate, filler, disintegrant in 1 / 2 formula amount, add binder solution, and make soft material;
[0075] ii) sieving the soft material to prepare wet granules, and drying;
[0076] iii) Whole granules, add disintegrants, glidants and lubricants in the remaining formulation amount, and mix to form a mixture;
[0077] iv) compressing the mixture obtained in step iii) to form tablets;
[0078] v) Optionally, coating the tablet obtained in step iv) to make a coated tablet.
[0079] The standards and definitions of suitable soft materials can be defined in accordance with the 6th edition of "Pharmacy" published by People's Health Publishing House in April 2008 (editor-in-chief Cui Fude).
[0080] In a specific embodiment of the present invention, the method includes: mixing alogliptin benzoate with mannit...
Embodiment 1
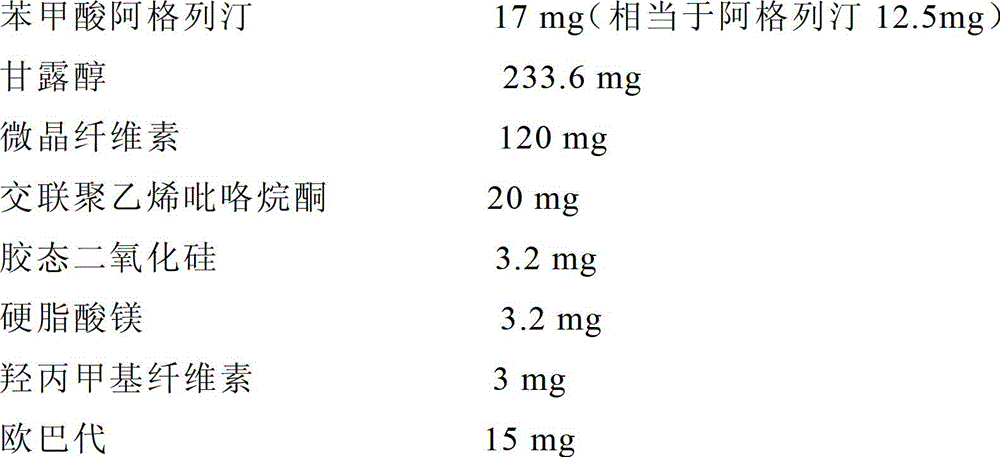
[0094] (1) Prescription (per tablet)
[0095]
[0096] (2) Preparation process
[0097] Mix the raw material with mannitol, then add microcrystalline cellulose and 1 / 2 formula amount of cross-linked polyvinylpyrrolidone, and add an appropriate amount (about 10ml / 100 tablets) of 3(w / v)% hydroxypropylmethylcellulose Aqueous solution, to obtain soft material, pass through 18-mesh sieve to granulate, dry at 60°C for 1 hour, pass through 18-mesh sieve for granulation, add the remaining cross-linked polyvinylpyrrolidone, colloidal silicon dioxide and magnesium stearate, and tablet to obtain Tablets are coated with Opadry.
Embodiment 2
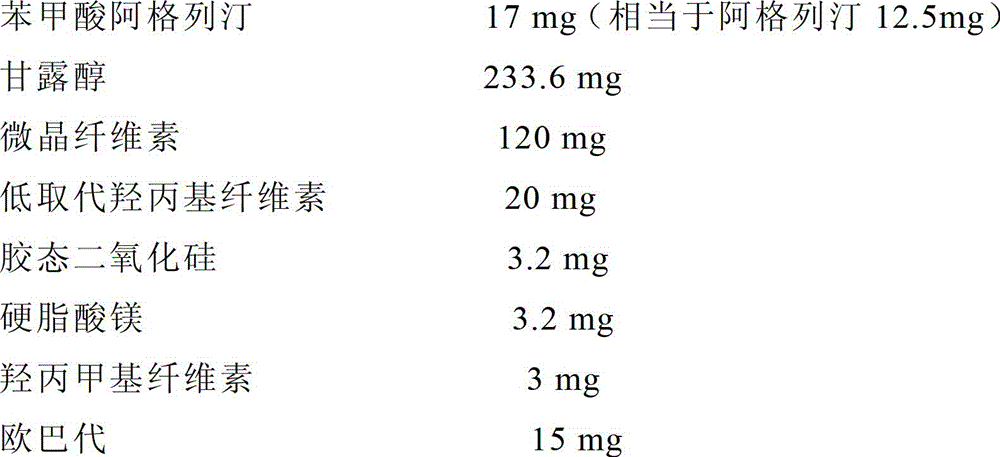
[0099] (1) Prescription (per tablet)
[0100]
[0101] (2) Preparation process
[0102] Mix the raw material drug with mannitol, then add microcrystalline cellulose and 1 / 2 formula amount of low-substituted hydroxypropyl cellulose, and add an appropriate amount of 3 (w / v)% hydroxypropyl methylcellulose aqueous solution to obtain soft material, granulated through a 18-mesh sieve, dried at 60°C for 1 hour, granulated through a 18-mesh sieve, added the remaining low-substituted hydroxypropyl cellulose, colloidal silicon dioxide and magnesium stearate, and compressed to obtain tablets. The resulting tablets were coated with Opadry.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com