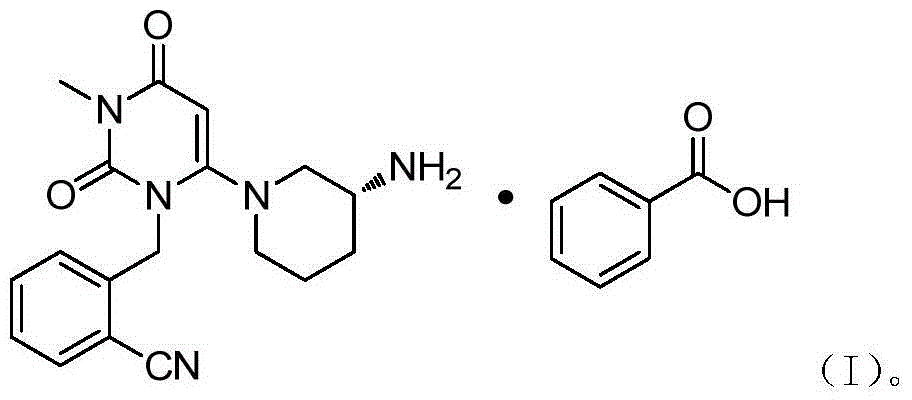
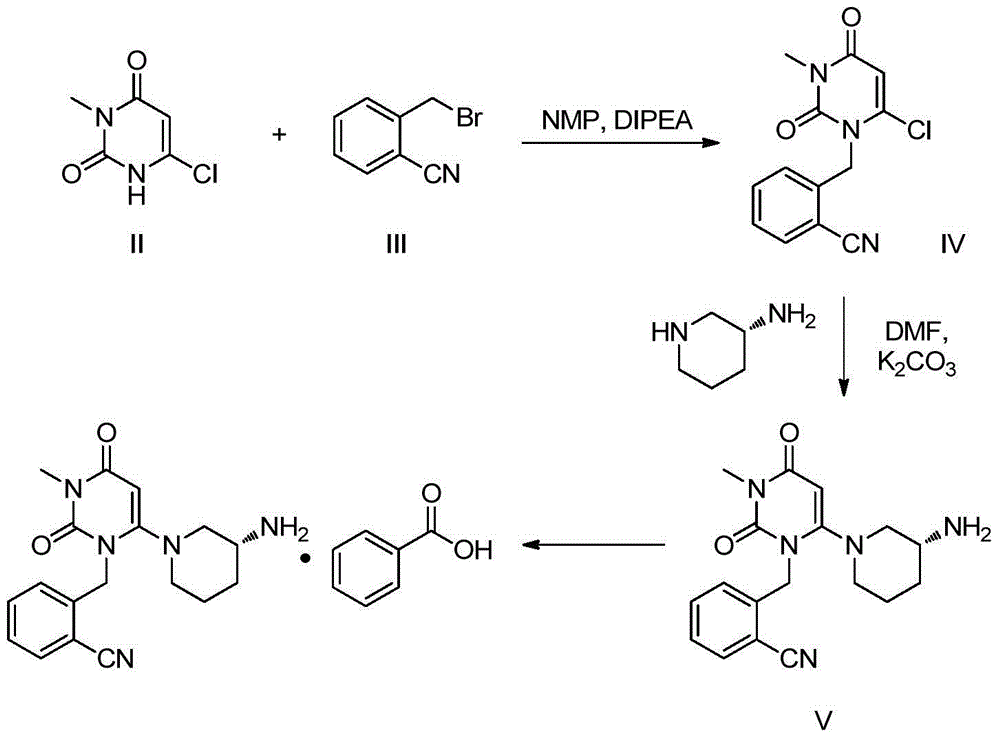
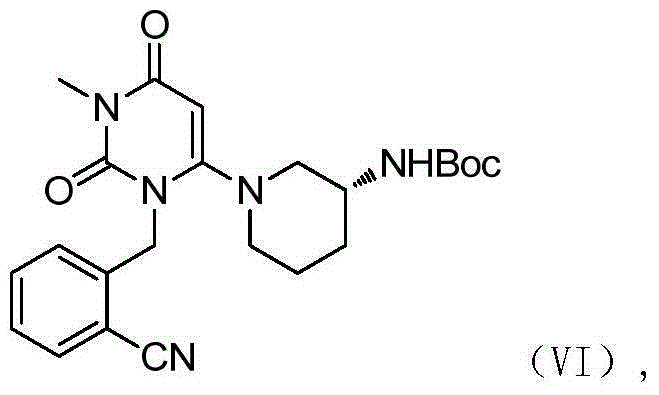
Method for preparing DPP-IV inhibitor
A -3-boc-, intermediate technology, applied in the direction of organic chemistry, etc., can solve the problems of complicated post-processing and low reaction yield, and achieve the effect of simple post-processing and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 (R)-tert-butyl (1-(3-(2-cyanobenzyl)-1-methyl-2,6-dioxo-1-1,2,3,6-tetrahydro -4-yl)piperidine-3-
[0040] base) preparation of tert-butyl carbamate
[0041]6-chloro-3-methyluracil (1kg), potassium carbonate powder (2.0kg), 2-cyanobenzyl bromide (2.34kg), N,N-dimethylacetamide (10kg), about 50 After reacting at 100 degrees Celsius for 4 hours, add potassium carbonate powder (1.0kg) to the reaction kettle, (R)-3-Boc-aminopiperidine (2.0kg) was stirred at 100 degrees Celsius for 4 hours, and after the reaction was completed, pour it into the reaction kettle Add a corresponding amount of water, cool to 50 degrees Celsius, stir for 1 hour, and centrifuge after cooling down to room temperature. After the filter cake is dried, it is washed with water and dried to obtain (R)-tert-butyl (1-(3-(2-cyano) Benzyl)-1-methyl-2,6-dioxo-1-1,2,3,6-tetrahydro-4-yl)piperidin-3-yl)carbamate tert-butyl ester, pink solid . Yield 94.5%, purity 99%.
Embodiment 2
[0042] Example 2 (R)-tert-butyl (1-(3-(2-cyanobenzyl)-1-methyl-2,6-dioxo-1-1,2,3,6-tetrahydro -4-yl)piperidine-3-
[0043] base) preparation of tert-butyl carbamate
[0044] In 6-chloro-3-methyluracil (1kg), potassium carbonate powder (2.5kg), 2-cyanobenzyl bromide (2.0kg), N,N-dimethylacetamide (15kg), about 85 After reacting for 2 hours at ℃, add potassium carbonate powder (1.5kg) and (R)-3-Boc-aminopiperidine (3.1kg) to the reaction kettle and raise the temperature of the system to 95 ℃, then stir at 95 ℃ 4 hours, add water (20kg) in reaction kettle after completion of reaction, cool down to 60 degrees centigrade, stir for 1 hour, centrifuge after being down to room temperature, wash with water after filter cake is dried, dry, obtain (R)-tert-butyl ( 1-(3-(2-cyanobenzyl)-1-methyl-2,6-dioxo-1-1,2,3,6-tetrahydro-4-yl)piperidin-3-yl ) tert-butyl carbamate, pink solid. Yield 93.2%, purity 98%.
Embodiment 3
[0045] Example 3 (R)-tert-butyl (1-(3-(2-cyanobenzyl)-1-methyl-2,6-dioxo-1-1,2,3,6-tetrahydro -4-yl)piperidine-3-
[0046] base) preparation of tert-butyl carbamate
[0047] In 6-chloro-3-methyluracil (1kg), potassium carbonate powder (1.8kg), 2-cyanobenzyl bromide (4.2kg), N,N-dimethylacetamide (13kg), about 65 After reacting at 85 degrees Celsius for 3 hours, add potassium carbonate powder (1.8kg) to the reaction kettle, (R)-3-Boc-aminopiperidine (4.0kg) was stirred at 85 degrees Celsius for 7 hours, and after the reaction was completed, pour it into the reaction kettle Add the corresponding amount of water, cool down to 60 degrees Celsius, stir for 1 hour, and centrifuge after cooling down to room temperature. After the filter cake is dried, it is washed with water and dried to obtain (R)-tert-butyl (1-(3-(2-cyano) Benzyl)-1-methyl-2,6-dioxo-1-1,2,3,6-tetrahydro-4-yl)piperidin-3-yl)carbamate tert-butyl ester, pink solid . Yield 94.1%, purity 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com