Method for measuring optical purity of R-alogliptin benzoate
A technology of alogliptin and optical purity, which is applied in the field of determination of optical purity of R-benzoic acid alogliptin, can solve the problem of low separation efficiency and achieve the effect of improving separation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
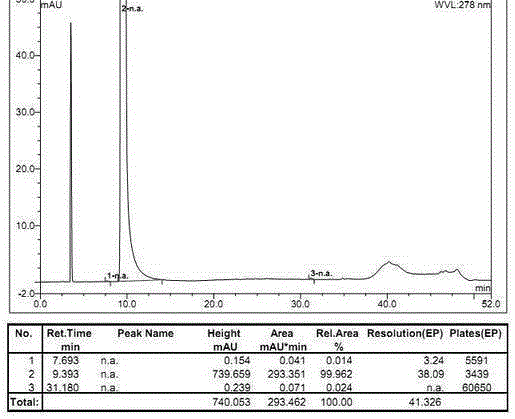
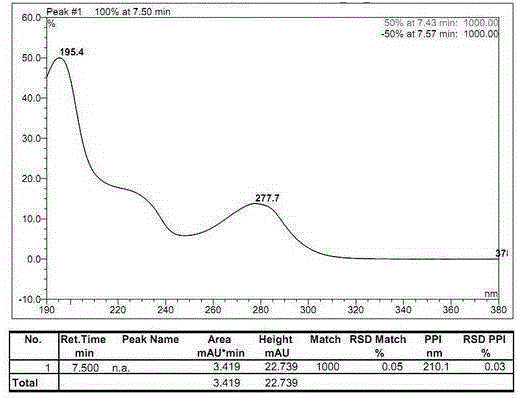
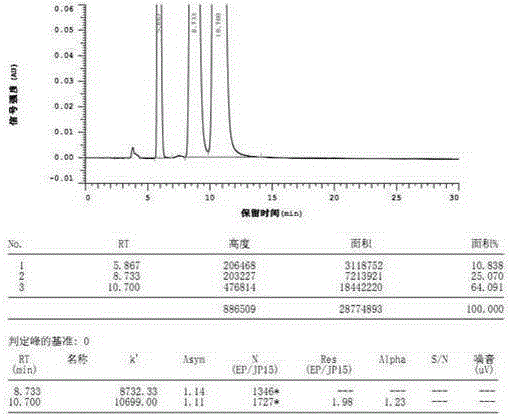
[0033] The determination of the optical purity of embodiment 1R-benzoic acid alogliptin sample
[0034] Instruments and reagents: high performance liquid chromatography (Hitachi, Japan; Thermo Fisher Scientific Co., Ltd.); C18 chromatographic column (250mm×4.6mm, 5μm; Thermo Fisher Scientific Co., Ltd.); AD-H chiral Chromatographic column (250mm×4.6mm, 5μm; Daicel Pharmaceutical Chiral Technology Co., Ltd.); constant temperature water bath (Jiangsu Jintan Kexing Instrument Factory); electronic constant speed stirrer (Shanghai Shensheng Technology Co., Ltd.).
[0035] n-Hexane (chromatographically pure, Tianjin Biaoshiqi Technology Co., Ltd.); absolute ethanol (chromatographically pure, Tianjin Biaoshiqi Technology Co., Ltd.); trifluoroacetic acid (chromatographically pure, Aladdin Reagent Company); diethylamine ( Analytical grade, Tianjin Guangcheng Chemical Reagent Co., Ltd.); R-benzoic acid alogliptin reference substance (purity ≧ 99.9%, Disha Pharmaceutical Group Drug...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com