Patents
Literature
284 results about "Perfluoroacetic Acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
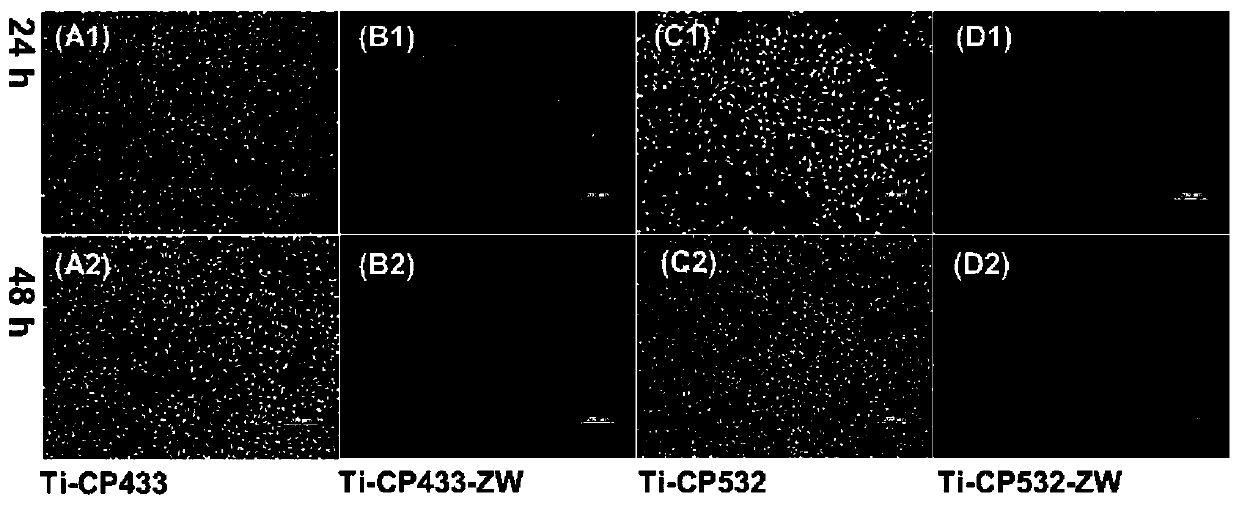
An electrostatically spun chitosan nano-fiber film for adsorption of heavy metal ions and a preparation method thereof
InactiveCN103285819ALarge specific surface areaHigh porosityOther chemical processesAlkali metal oxides/hydroxidesFiberAdsorption equilibrium
The present invention relates to an electrostatically spun chitosan nano-fiber film for adsorption of heavy metal ions and a preparation method thereof. The preparation method comprises the following steps of: (1) dissolving chitosan with a degree of deacetylation of between 70% to 80% and a molecular weight of between 200,000 to 300,000 in trifluoroacetic acid, and obtaining an uniformly dissolved spinning solution after ultrasonic treatment for 1-2h; (2) adding the above spinning solution into an electrostatic spinning device and preparing chitosan nano-fiber film through an electrostatic spinning method; and (3) immersing the obtained chitosan nano-fiber film in a 1mol / L of NaHCO3 solution for 24h at room temperature, and obtaining neutralized chitosan nano-fiber film after washing and drying. The electrostatically spun chitosan nano-fiber film provided by the invention has the characteristics of a large specific surface area, a high porosity, abundant inside pores, etc.; the contact area with metal ions and the absorption capacity can be increased and the time to reach adsorption equilibrium is shortened, thus the electrostatically spun chitosan nano-fiber film can effectively adsorb heavy metal ions in wastewater and can be biodegradable, causing no environmental pollution.
Owner:SUZHOU ZHENGYECHANG INTELLIGENT TECH
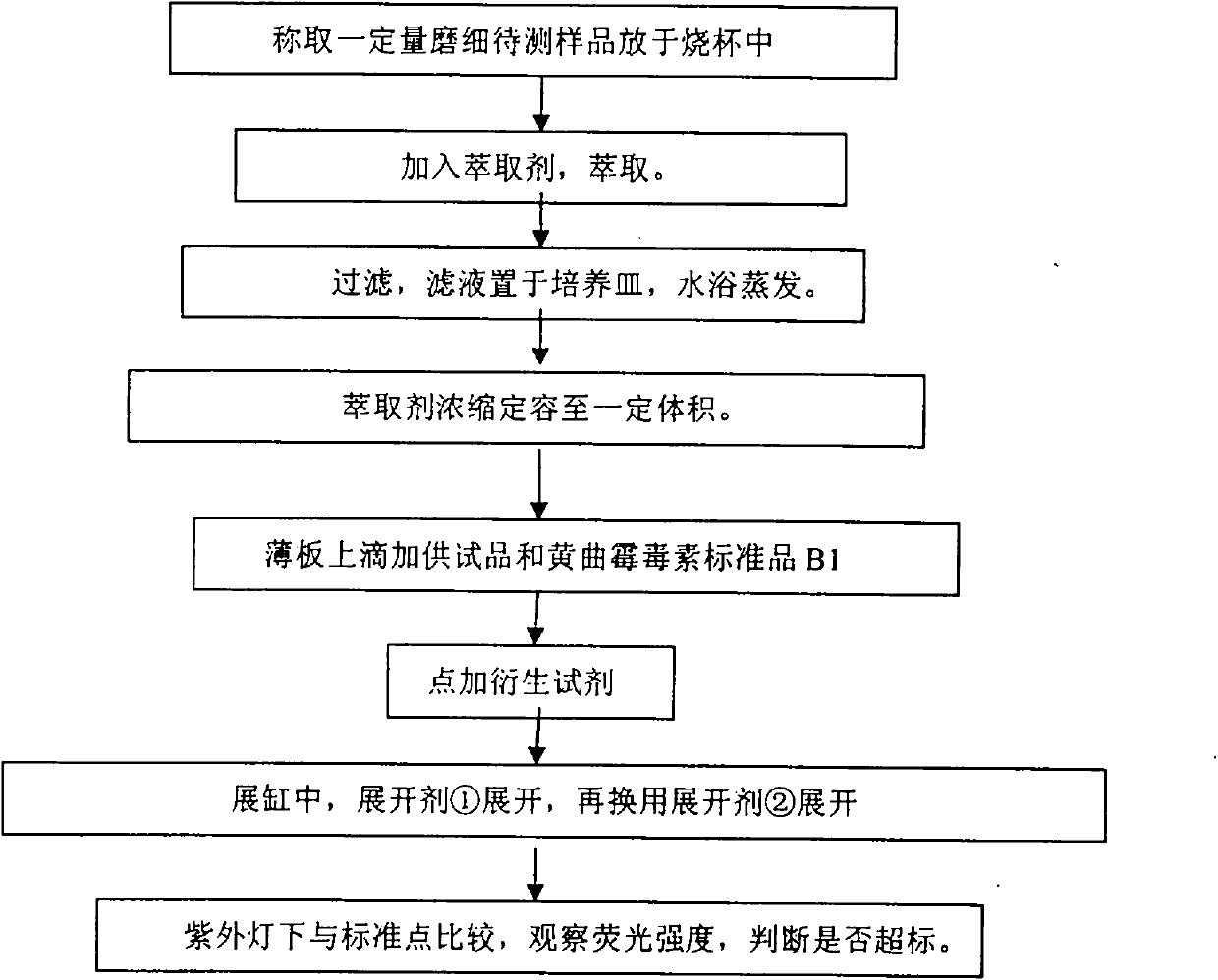
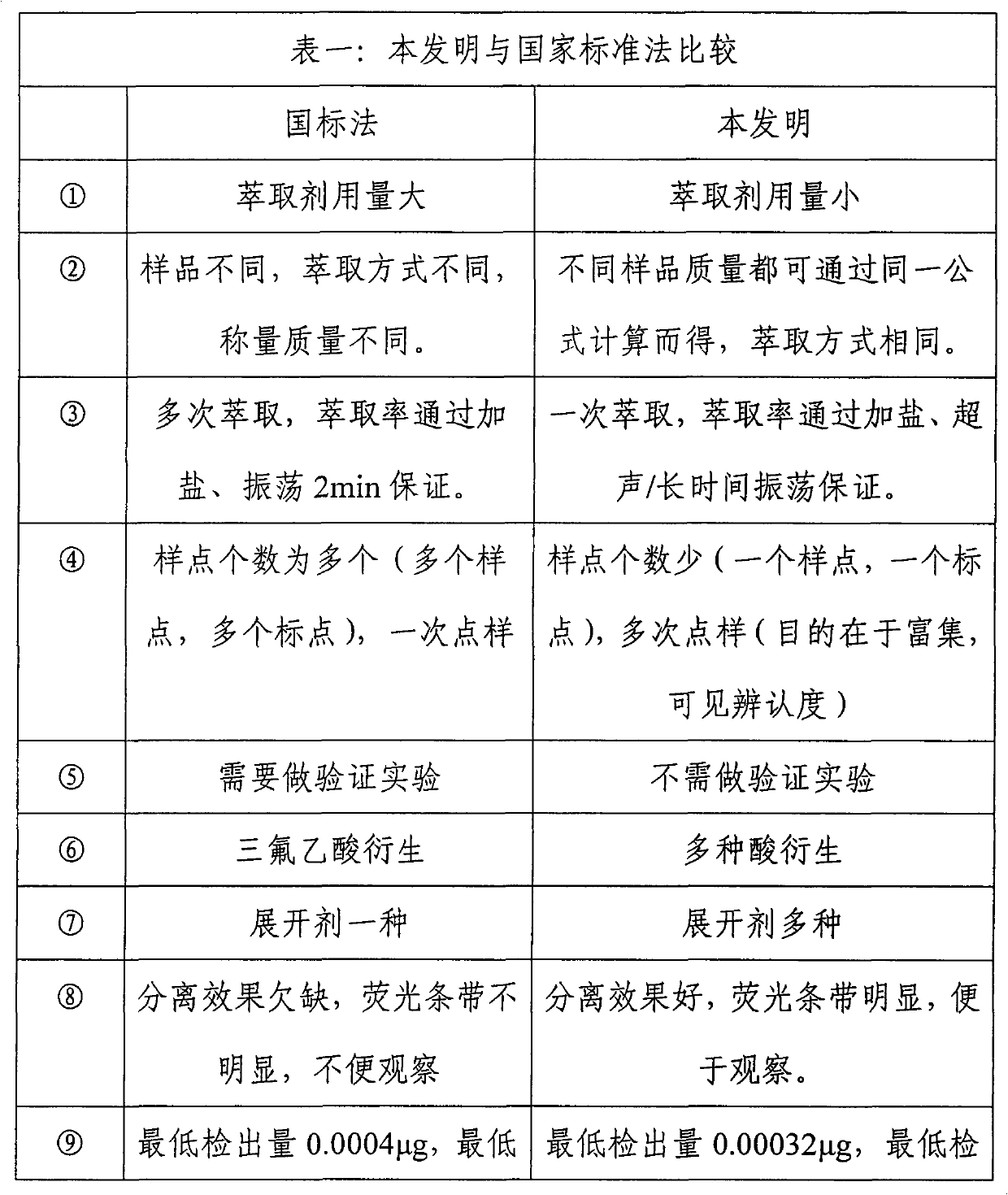
Method for rapidly detecting content of aflatoxin
InactiveCN101949854AReduce dosageReduce pollutionMaterial analysis by observing effect on chemical indicatorFluorescence/phosphorescencePerfluoroacetic AcidSilica gel
The invention discloses a method for rapidly detecting the content of aflatoxin. The method comprises the following steps of: after smashing a sample to be detected, putting the sample powder into a beaker, and adding an extractant for shaking or performing ultrasonic extraction; filtering, performing water bath evaporation on a filtrate, and metering the volume to obtain a test sample; respectively pointwise adding the test sample and an aflatoxin B1 standard substance which are commensurate on a thin-layer silica gel plate, pointwise adding derivative reagents into sampling points, and respectively developing by developers; and comparing the fluorescence intensity of the test sample and the aflatoxin B1 standard substance under an ultraviolet lamp to judge whether the content of the aflatoxin in the test sample exceeds the standard. The method for rapidly detecting the content of the aflatoxin has the advantages of small solvent amount, simple sample treatment and short measurement time; the method does not need a professional, only needs small apparatus, and is particularly suitable for rapid field detection; and the method effectively avoids the harm and the pollution of trifluoroacetic acid to operators and the environment, improves the method sensitivity, and has the lowest detectable amount of 0.00032 mu g and the lowest detection limit of 3 mu g / kg.
Owner:云南健牛环境监测有限公司
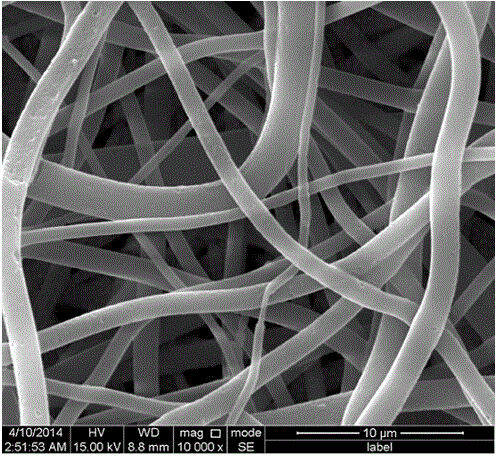
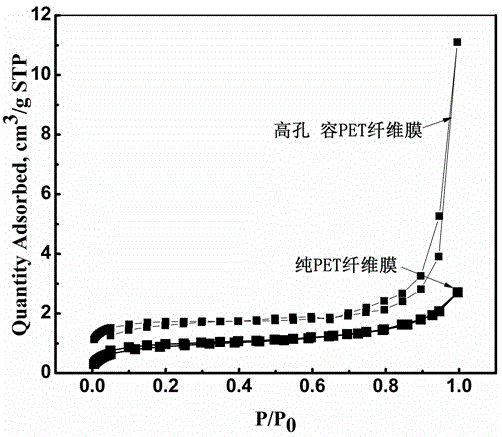
Preparation method of porous micro-nano PET fibers
ActiveCN104562292ALarge specific surface areaHigh porosityFilament/thread formingFiltration separationMicro nanoFiber
The invention discloses a preparation method of porous micro-nano PET fibers. The preparation method comprises the following steps: using PET and polymer as raw materials, dissolving in a mixed solvent of trifluoroacetic acid and dichloromethane, and stirring and dissolving at room temperature to obtain a homogeneous solution; performing electrostatic spinning to obtain micro-nano composite fibers; placing the composite fibers in an extracting agent to remove the polymer until the sample is constant in weight, rinsing several times with distilled water, and drying to obtain the porous micro-nano PET fibers. The porous micro-nano PET fibers have a higher specific surface area, and improve the porosity and specific surface area on the basis of maintaining advantages of conventional electrostatic spinning PET; the preparation of porous structure can be completed only by the extraction process, so that the method is simple and easy to perform. Surfaces and inner parts of the fibers have independent through holes, and the size and number of holes can be controlled according to a mass ratio of PET to another added polymer, so that the porous micro-nano PET fibers with different specific surface areas and porosities can be obtained and are applied to the filtration field to better improve the micro-nano-particles interception effect.
Owner:YIZHENG XINGHAI CHEM FIBER
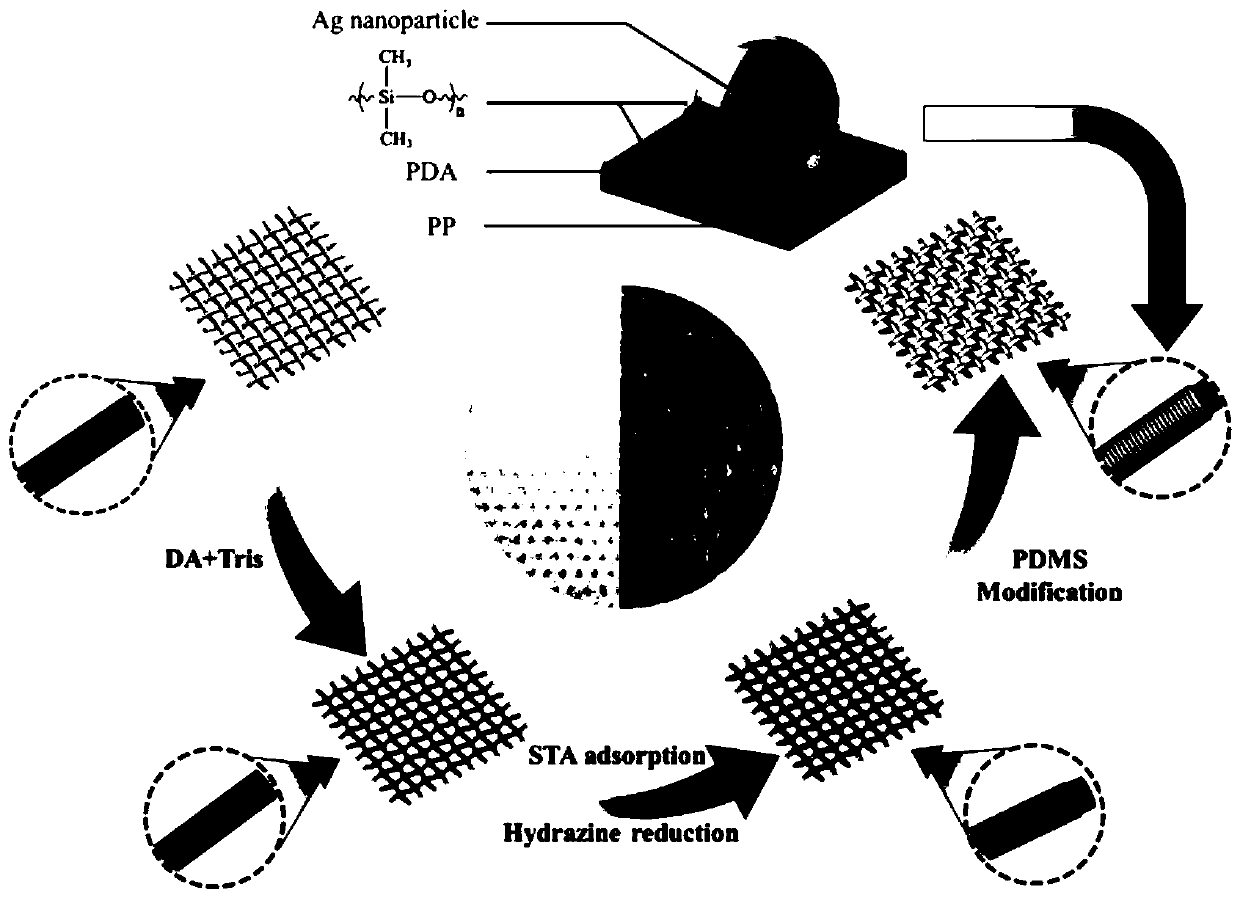
Superhydrophobic conductive composite fabric with electromagnetic shielding performance and preparation method thereof
ActiveCN109722900AEasy to operateReduce energy consumptionMagnetic/electric field screeningFibre typesElectromagnetic shieldingPolypropylene
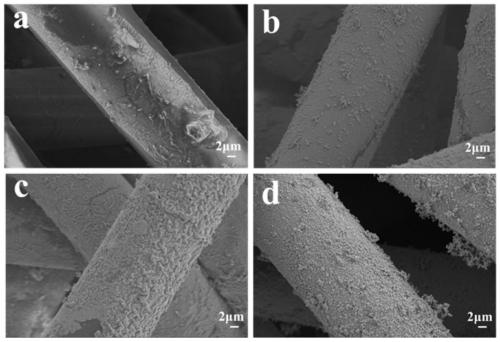
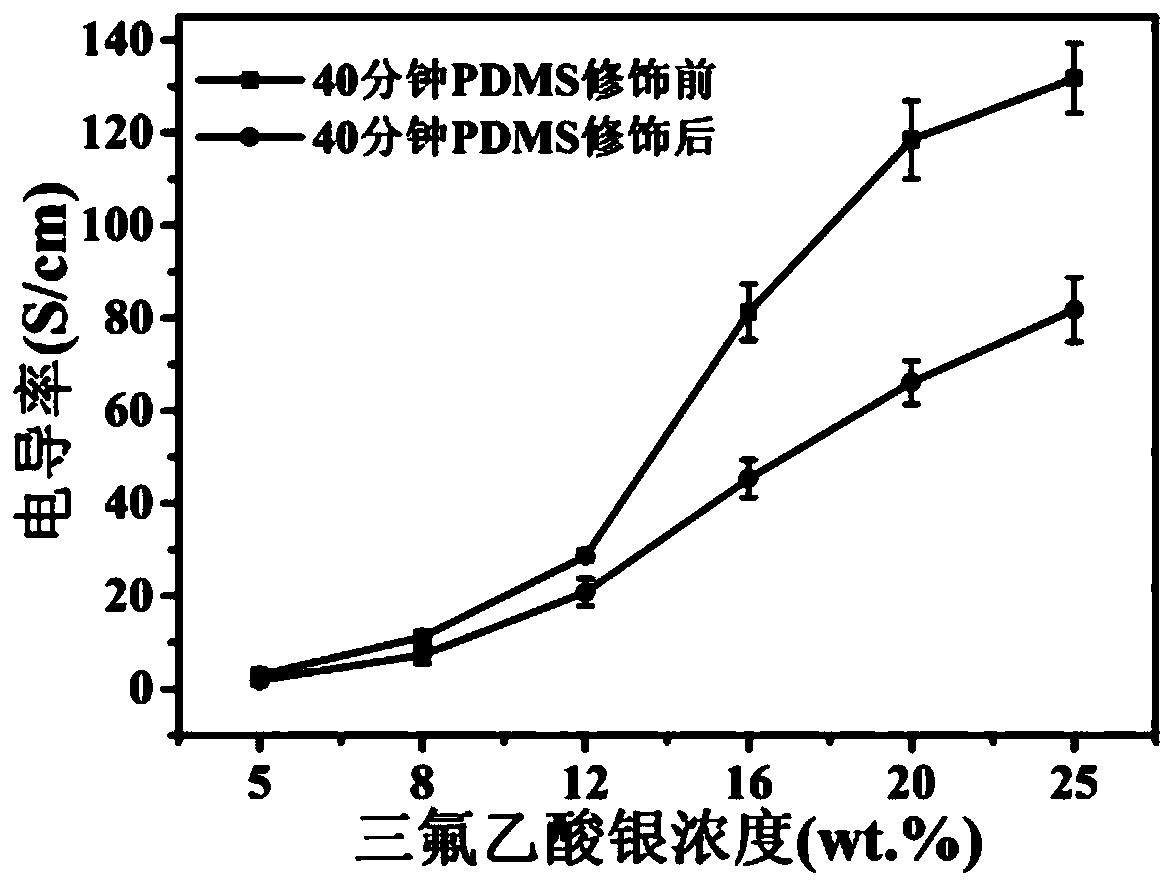
The invention discloses a superhydrophobic conductive composite fabric with the electromagnetic shielding performance and a preparation method thereof. The method comprises the steps that a polypropylene non-woven fabric is immersed in a dopamine solution, a self-polymerization reaction is conducted to obtain polypropylene non-woven fabric with the surface modified with polydopamine, then the fabric is immersed in a silver trifluoroacetate ethanol solution, a hydrazine hydrate solution is added for reduction, and finally the fabric is immersed in a PDMS n-heptane solution to obtain the superhydrophobic conductive composite fabric with the electromagnetic shielding performance. The conductivity of the superhydrophobic conductive composite fabric is up to 80 S / cm, the 72-dB electromagnetic shielding efficiency is achieved, the superhydrophobic performance is achieved, and the contact angle can reach 152 degrees, and excellent electromagnetic shielding performance can still be kept through multiple abrasion and winding and acid corrosion for 20 hours, and the fabric has the excellent abrasion resistance and corrosion resistance performance.
Owner:YANGZHOU UNIV
Method for measuring 2-furfural in beer by using high performance liquid chromatography
ActiveCN101762647AImprove retentionImprove accuracyComponent separationChromatographic columnChemistry
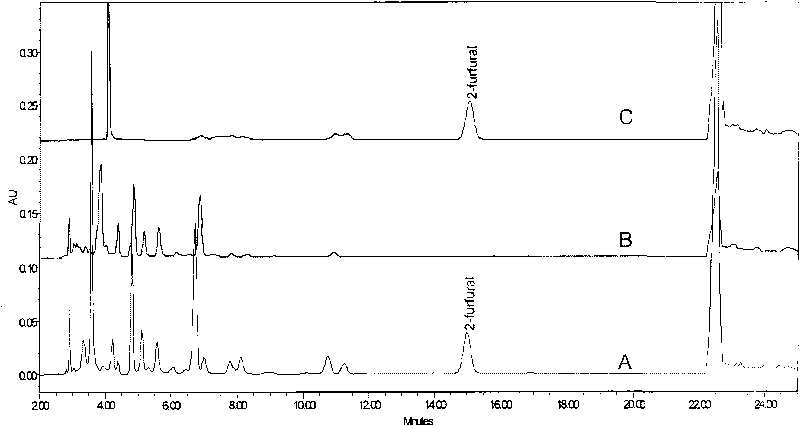
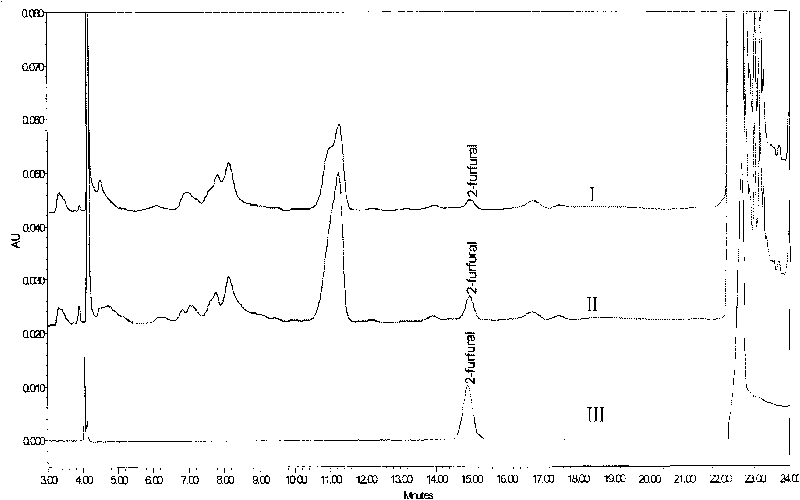
The invention provides a method for measuring 2-furfural in beer by using a high performance liquid chromatography. The method comprises the steps of: sample preparation, pre-treating program for the beer to be measured, gradient elution program, and comparison detecting program of beer and sample spectrogram. In the pre-treating program for the beer to be measured,the solid-phase extraction column PEP-SPE is adopted to maintain the beer 2-furfural. In the gradient elution program, a mobile phase A is 0.01% of trifluoroacetic acid aqueous solution, the mobile phase C is ultrapure water, the mobile phase D is acetonitrile, wherein the ratio of the mobile phase A is kept 95% and the ratio of the mobile phase D is kept 5% in 0 to 16 minutes; the mobile phase C is 40% and the mobile phase D is 60% in 16 to 16.5 minutes; the ratio is maintained for three minutes to thoroughly wash a chromatographic column; the ratio of the mobile phase A is recovered to 95% and the ratio of the mobile phase D is recovered to 5% in 19.5 to 20.0 minutes; the ratio is kept for 8 minutes; the whole gradient elution program is finished at the 28th minutes. The flow speed in each step is 1.0 ml per min and the ratio conversion of the mobile phase is performed in gradient linear mode. The method of the invention can efficiently purify beer and collect the 2-furfuralon the Waters Alantis C18 (250mm*46mm) chromatographic column, 0.01% of trifluoroacetic acid and acetonitrile are treated with gradient elution program, and the 2-furfural in beer can be measured with a wavelength of 280nm. The recycling ratio of the method is 99.8% to 100.2%, the repeatability RSD (n equals to 6) is equal to or less than 2.2% and the minimum detection limit is 5 ug / l.
Owner:TSINGTAO BREWERY
Scale and corrosion inhibitor and application of scale and corrosion inhibitor in salt draining closed conduit of saline and alkaline land
ActiveCN105016493AReduce corrosion rateGood scale resistanceScale removal and water softeningBenzoic acidPerfluoroacetic Acid
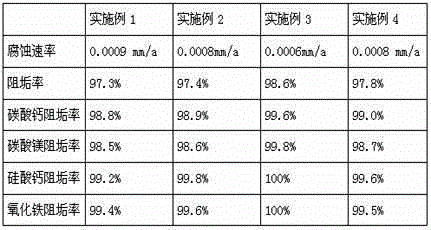
The invention provides a scale and corrosion inhibitor and application of the scale and corrosion inhibitor in a salt draining closed conduit of a saline and alkaline land. The scale and corrosion inhibitor comprises the components in parts by weight: 26-29 parts of ursolic acid, 15-18 parts of rosmarinic acid, 4-6 parts of trifluoroacetic acid, 6-8 parts of bis-1,6-hexylidenetriamine pentamethylene sodium phosphonate, 3-5 parts of thionothiolic acid, 1-3 parts of mannitol, 8-12 parts of polyepoxysuccinic acid, 6-9 parts of sodium benzotriazole, 2-4 parts of propyl hydroxybenzoate and 50-60 parts of water, wherein the purity of ursolic acid is 98%; the purity of rosmarinic acid is 95%; and the solid content of sodium benzotriazole is 40%. By using the scale and corrosion inhibitor provided by the invention, the corrosion rate, which is 0.0006-0.0009 mm / a, of the salt draining closed conduit is obviously reduced, and the service life of the salt draining closed conduit can be prolonged by 10 years.
Owner:WEIFANG YOURONG IND
Bamboo charcoal/carbon aerogel composite material and preparation method thereof
InactiveCN102430369AHigh specific surface areaLarge specific surface areaColloidal chemistry detailsToxic materialNitrogen gas
The invention discloses a method for preparing bamboo charcoal / carbon aerogel composite material, comprising the following steps: 1) mixing m-dihydroxybenzene with formaldehyde, dissolving in water, and stirring to obtain mixed liquid A; 2) adding the bamboo charcoal to the mixed liquid A and sealing; firstly standing at room temperature, then standing at 70-80 DEG C, and filtering to obtain mixture B; 3) putting the mixture B in trifluoroacetic acid liquor of which the mass concentration is 0.4-0.6% to soak, then taking outside and putting in acetone to soak, wherein the acetone is changed every day; and 4) putting the mixture after being dried in a nitrogen atmosphere furnace, insulating at 800-1000 DEG C for 1-3 hours, and obtaining the bamboo charcoal / carbon aerogel composite material. The composite material can be widely applied to industries for purifying air, processing waste water, processing toxic waste water, chemical catalyst carrier, purifying tail gas of cars, preparing sugar and wine, and preparing food.
Owner:ZHEJIANG UNIV
Catalyst for copolymerization of carbon dioxide and epoxy compounds, preparation method and applications thereof
InactiveCN101565502ASimple preparation stepsLow costCobalt organic compoundsEpoxyBulk polymerization
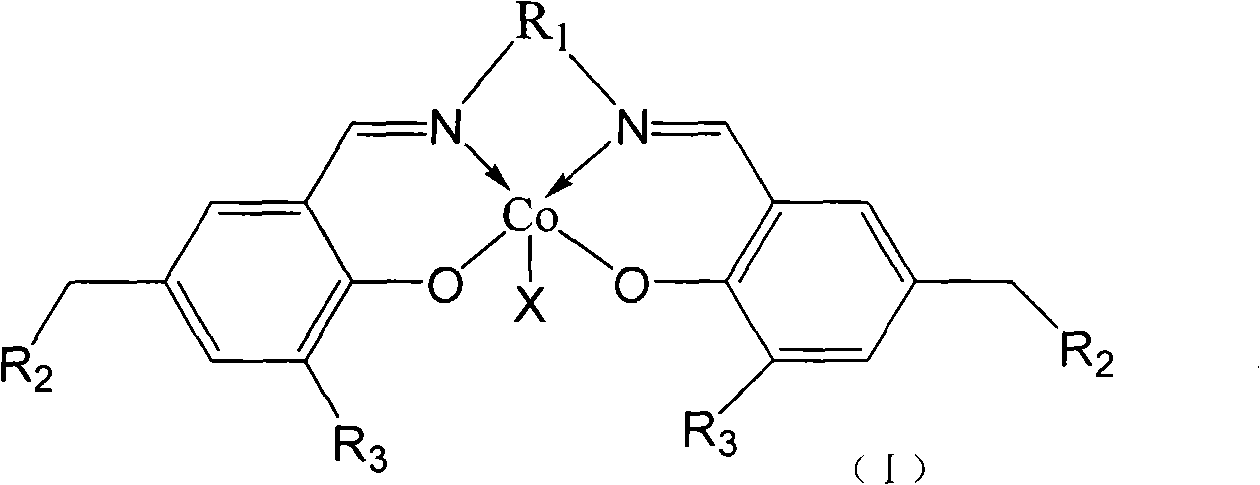
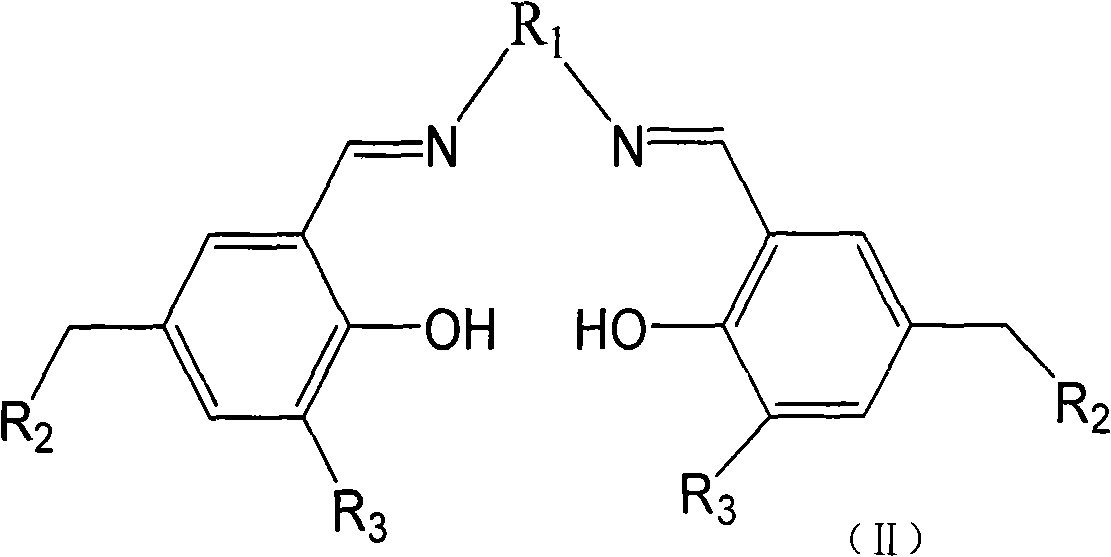
The invention relates to a catalyst for copolymerization of carbon dioxide and epoxy compounds, a preparation method and applications thereof. The structural formula of the catalyst is compound (I) at the right side, wherein X refers to halogen, nitrate radical, azide group or trifluoroacetic acid group; R1 is 1, 2-site disubstituted cyclohexyl or 1, 2-site disubstituted 1, 2-diphenyl-ethyl; R2 is N substituted pyrrolidine, piperidyl, morpholinyl, dibutylamine or 4- methylamino-pyridyl; R3 is tert-butyl group. The preparation method of the catalyst for the copolymerization of carbon dioxide and epoxy compounds comprises the two preparation steps of a ligand and the catalyst. The catalyst preparation method has simple preparation steps, low cost and good catalysis efficiency and product selectivity. Under the noumenon polymenrism or the solution polymenrism, the carbon dioxide and epoxy compounds are polymerized under the lower polymenrism of carbon dioxide (optimal pressure is 30 atm) and simultaneously the copolymer with the content of the polycarbonate unit of more than 98% and the catalyst with the catalytic efficiency of 60-214g polymer / g are obtained.
Owner:HEBEI UNIV OF TECH
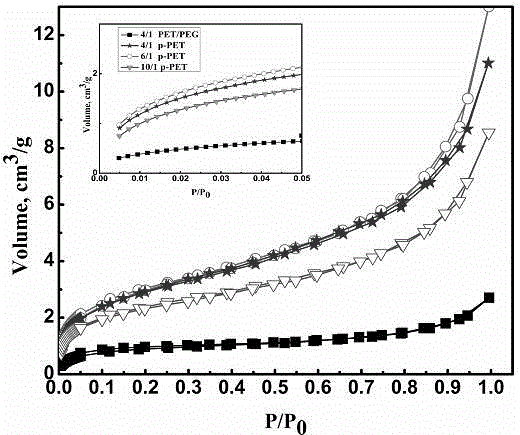
Preparation method for micro and nano PET fiber with large pore volumes
ActiveCN105019142AHigh pore volumeLarge specific surface areaFilament/thread formingNon-woven fabricsFiberPolyethylene terephthalate glycol
The invention discloses a preparation method for micro and nano PET fiber with large pore volumes. Polyethylene terephthalate (PET) and a porous material with large specific surface area are used as starting material. Trifluoroacetic acid and dichloromethane are used as mixed solvent to prepare a mixed solution with certain concentration. Complex fiber with a micro and nano structure is made by electrostatic spinning. The complex fiber is etched in a certain aqueous alkali, then washed repeatedly with distilled water and dried. Thus, the micro and nano PET fiber with large pore volumes is obtained. Due to partially exposed porous carbon fiber at the surface of the fiber and high porosity of electrostatic spinning, the prepared electrospinning has large pore volumes. The micro and nano PET fiber with large pore volumes will have many applications in the fields of catalysis and adsorption and can be used as adsorbent material or catalyst carrier. The preparation condition of the method is mild and the method is easy and feasible.
Owner:深圳市和龙电子有限公司
Preparation method and application of metal organic framework MOF-808 film-based functional sandwich material
ActiveCN112688021AHigh specific capacityImprove cycle stabilityElectrode carriers/collectorsLi-accumulatorsLithium–sulfur batteryPerfluoroacetic Acid
The invention relates to a preparation method and application of a metal organic framework MOF-808 film-based functional interlayer material. The method comprises the following steps: dissolving zirconium oxychloride octahydrate and trimesic acid in deionized water, performing magnetic stirring for 30-60 minutes at normal temperature, then adding trifluoroacetic acid, and carrying out ultrasonic treatment for 30-60 minutes to obtain a synthetic solution; and then vertically immersing a carbon nanotube (CNT) film wafer in the synthetic liquid, and growing for 3-5 hours at the temperature of 110-130 DEG C to obtain the MOF-808 / CNT film. The MOF-808 / CNT film interlayer material obtained by the invention is simple to operate, easy to amplify and suitable for industrial production; when the material is used as an interlayer material between a positive electrode and a diaphragm in a lithium-sulfur battery, the performance of the lithium-sulfur battery can be remarkably improved, the reversible capacity of the lithium-sulfur battery can reach 1292mAh g <-1 >, and the cycle performance is stable.
Owner:HEBEI UNIV OF TECH
Filtering membrane material applied to air purification and preparation method thereof
ActiveCN105771694AEasy to separateImprove mechanical propertiesSemi-permeable membranesDispersed particle separationFiberPolyamide
The invention discloses a filtering membrane material applied to air purification and a preparation method thereof. The filtering membrane material consists of a non-woven fabric substrate and a filtering layer, wherein the filtering layer is prepared from the following raw materials in parts by weight: polytetrafluoroethylene, polyglycerol polyricinoleate, polyamide, graphene oxide, nano cellulose, polyhydroxybutyrate, an antibacterial agent, maifanite powder, barium carbonate, zeolite powder, bamboo fibers, trifluoroacetic acid and distilled water. According to the filtering membrane material prepared by the preparation method, the tensile strength is 5.8MPa to 7.1MPa, the air flux is 1,491L / (m<2>*h) to 1,562L / (m<2>*h), the membrane interception rate is higher than 95.6%, and excellent separability, mechanical properties and durability are displayed, so that the filtering membrane material can be extensively applied to the field of air purification and is wide in market prospect.
Owner:ZHEJIANG DONGMENG MEDICAL EQUIP CO LTD
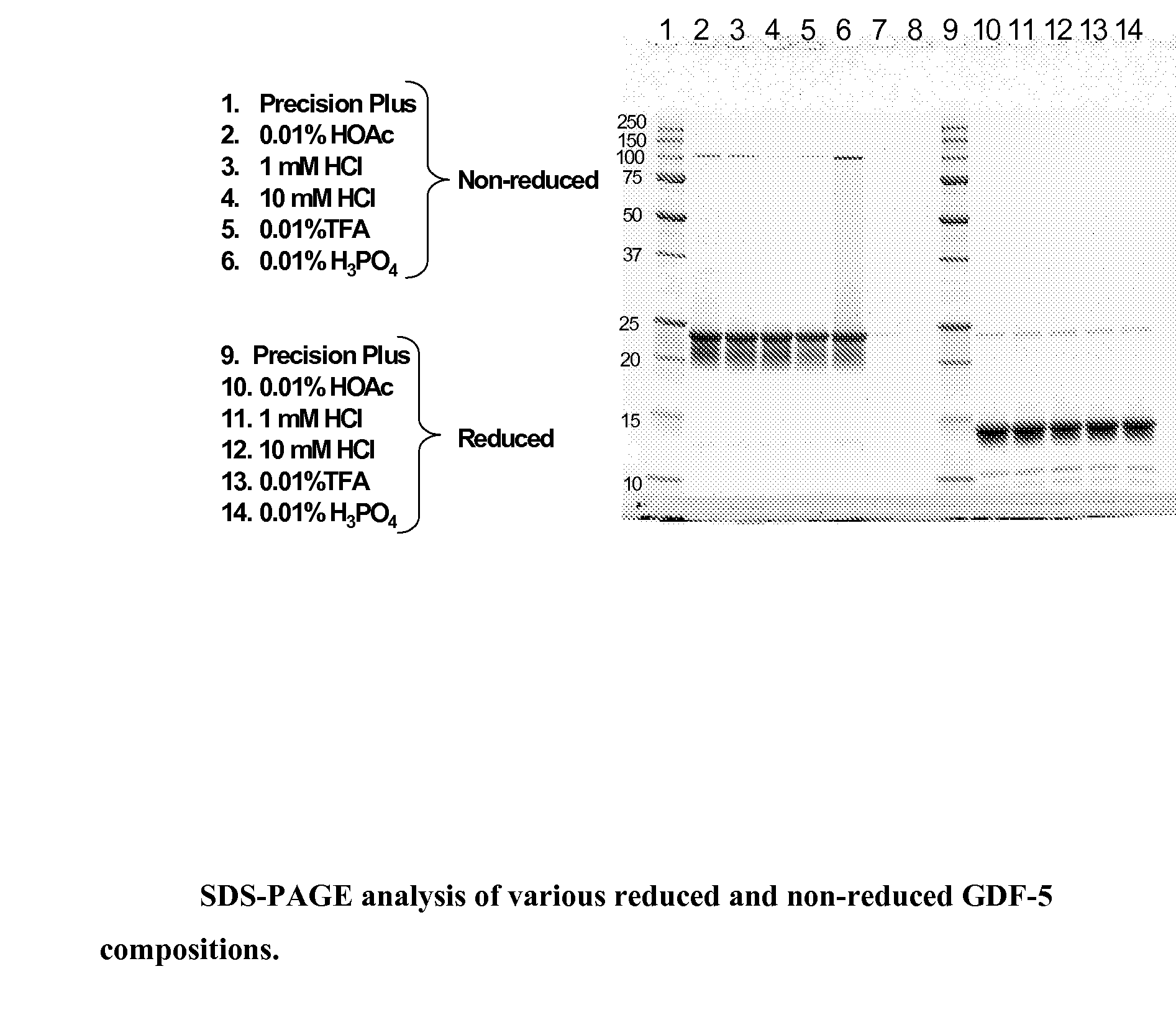
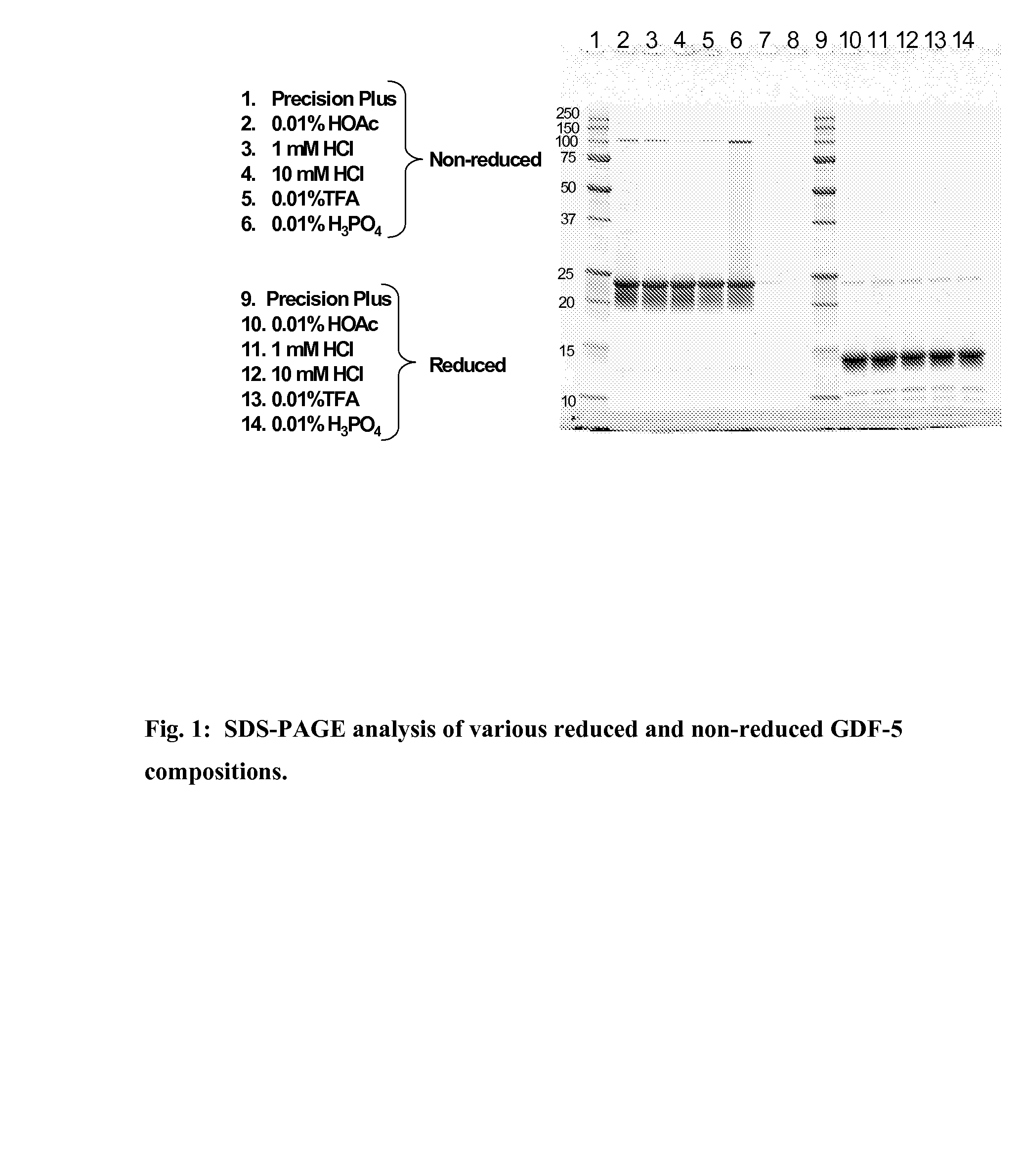
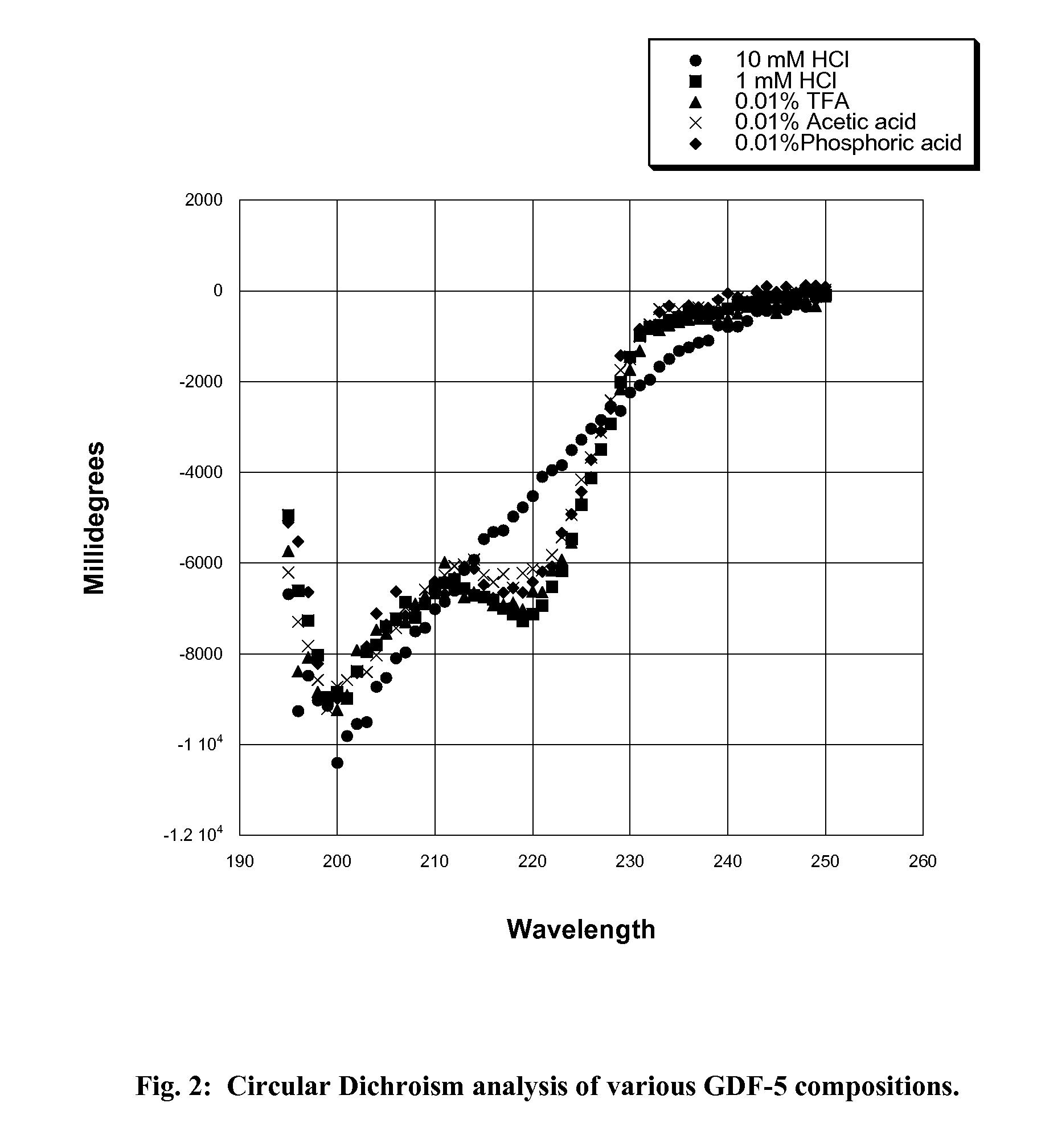
Gdf-5 protein storage
InactiveUS20090043078A1Improve stabilityWithout adversely affecting the solubility of the proteinPeptide/protein ingredientsInorganic non-active ingredientsTrifluoroacetic acidAqueous solution
Improved compositions and methods are provided for stabilizing a solution of bone morphogenetic protein. The compositions comprise an aqueous solution of GDF-5 and a biocompatible acid, such as hydrochloric, acetic, phosphoric, or trifluoroacetic acid, wherein the solution has a pH of from about 3.0 to about 3.6, thereby providing for improved stability of the GDF-5 protein during handling and prolonged storage at reduced temperatures.
Owner:ADVANCED TECH & REGENERATIVE MEDICINE
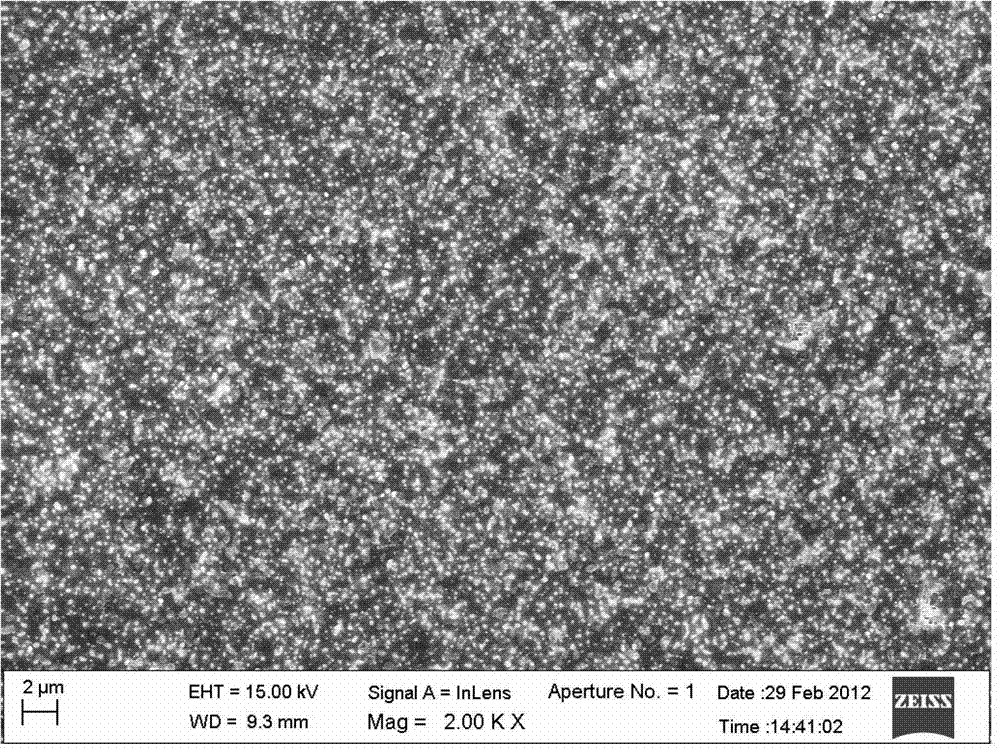
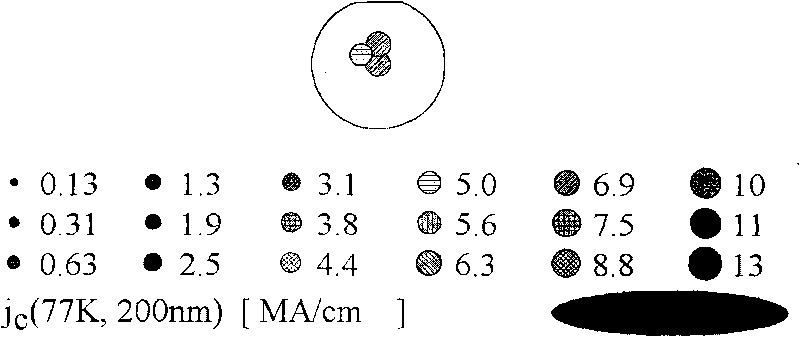
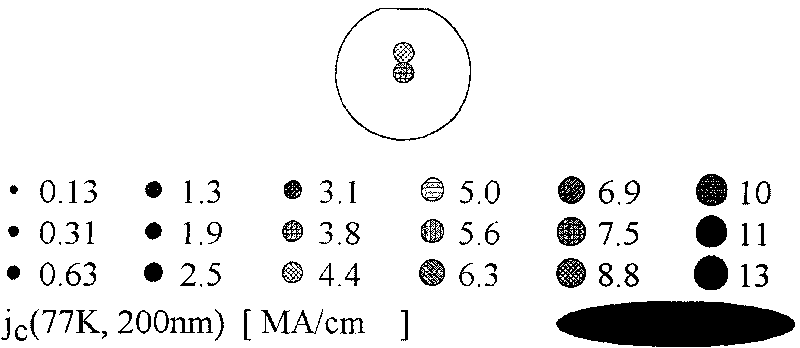
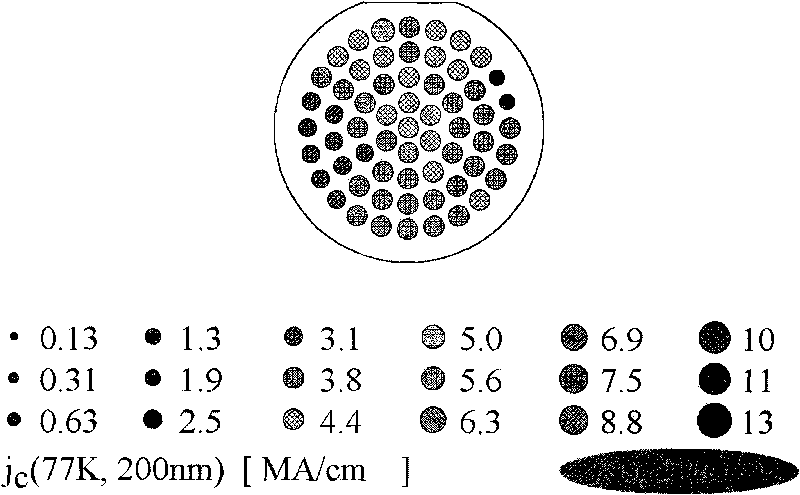
YBCO superconductive film with multi-layer composite structure and preparation method of film
ActiveCN102931338ARelaxation stressAvoid crackingSuperconductor detailsSuperconductor device manufacture/treatmentPerfluoroacetic AcidMethanol
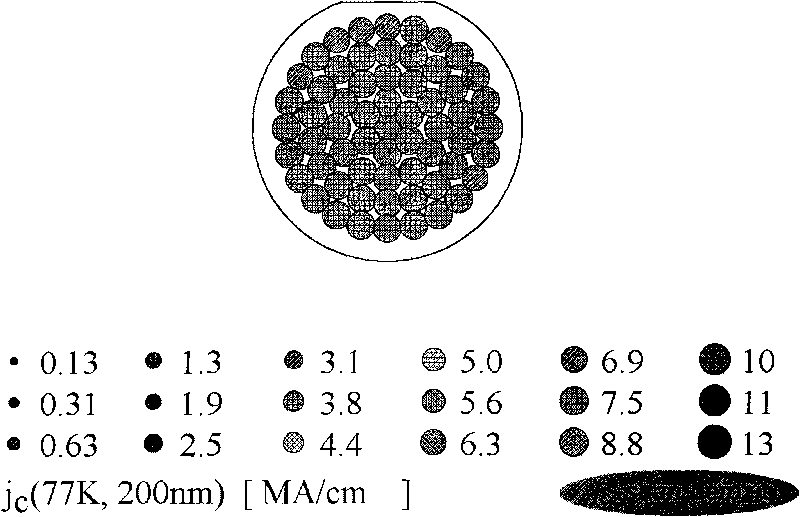
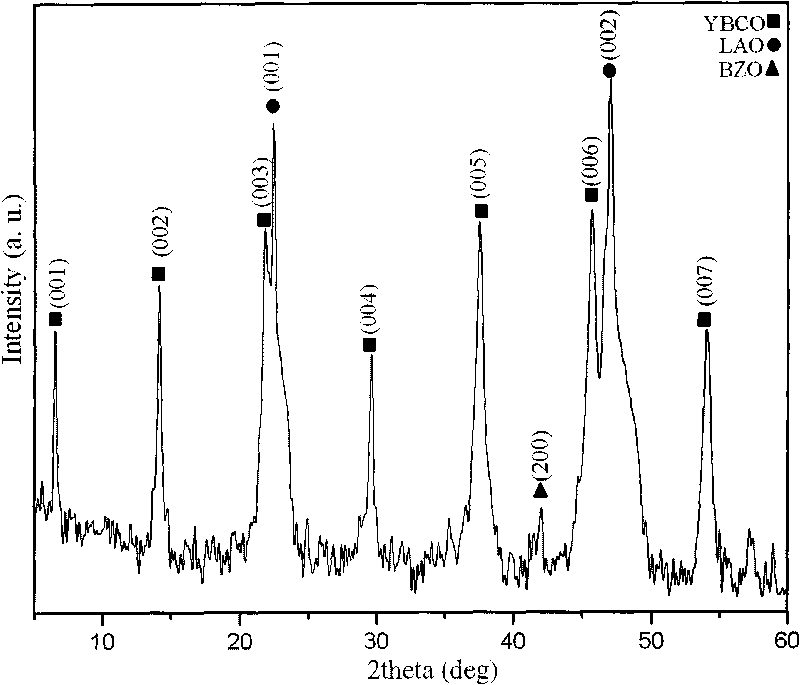
The invention provides an YBCO superconductive film with a multi-layer composite structure and a preparation method of the film. The method comprises the following steps of: firstly, mixing and dissolving ytterbium acetate hydrate, barium acetate and copper acetate in aqueous solution of trifluoroacetic acid according to the proportion; stirring evenly, evaporating the dissolvent to dryness under vacuum condition to obtain gel; adding methyl alcohol, stirring evenly and evaporating the dissolvent to dryness to obtain the gel; then, adding methyl alcohol to prepare precursor solution; coating the precursor solution on a substrate; carrying out low-temperature thermal treatment on the coated film firstly to decompose the trifluoro acetate; then, carrying out high-temperature thermal treatment to obtain a tetragonal YBCO film; coating the precursor solution of the titanium acetylacetonate on the the YBCO film, and carrying out high-temperature thermal treatment; and orderly coating the precursor solution of Y, Ba and Cu and the precursor solution of the titanium acetylacetonate on the film, and carrying out corresponding thermal treatment to prepare the YBCO superconductive film with the thickness of seven layers, wherein the structure of the YBCO superconductive film is YBCO / BaTiO3 / YBCO / BaTiO3 / YBCO / BaTiO3 / YBCO. The critical electric current density of the YBCO thick film reaches 4.0MA / cm<2> in the null field, thus, current-carrying capability of the YBCO thick film is improved greatly.
Owner:SUZHOU NEW MATERIAL INST +2
Method for preparing high temperature superconducting thin film by chemical process
ActiveCN101752035AIncrease the critical current densitySuperconductors/hyperconductorsSuperconductor devicesRare-earth elementEvaporation
The invention provides a method for preparing high temperature superconducting thin film by chemical process. The method comprises the following steps: firstly preparing a precursor solution, by mixing Y(CH3COO)3, RE(CH3COO)3, Ba(CH3COO)2 and Cu(CH3COO)2 with ratio of (Y+RE) to Ba to Cu equaling to 1:2:3 (wherein RE is Nd, Sm, Eu, Gd and Dy) and dissolving in 20-30 mol percent aqueous trifluoroacetic acid solution; stirring to be uniform, performing vacuum drying by evaporation to the solvent to obtain gel; then adding methanol, stirring to be uniform, performing drying by evaporation to the solvent to obtain gel; then adding proper amount of methanol to prepare the precursor solution with the total concentration of metal ions of Y, RE, Ba and Cu being 0.8-3.0mol / L; then coating the precursor solution on a substrate; performing low temperature heat treatment at 400-410 DEG C to the coated thin film to decompose trifluoroacetic acid salt; and finally performing high temperature heat treatment at 800-850 DEG C and annealing process at 490-510 DEG C to form YBCO thin film doped with rare-earth element.
Owner:GRIMAT ENG INST CO LTD
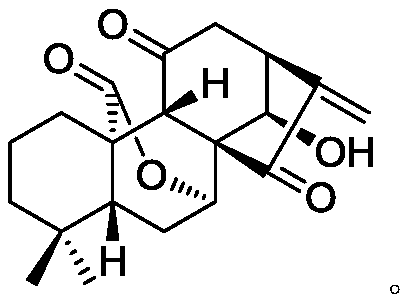
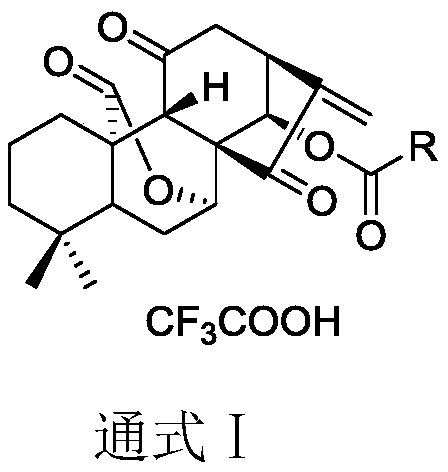
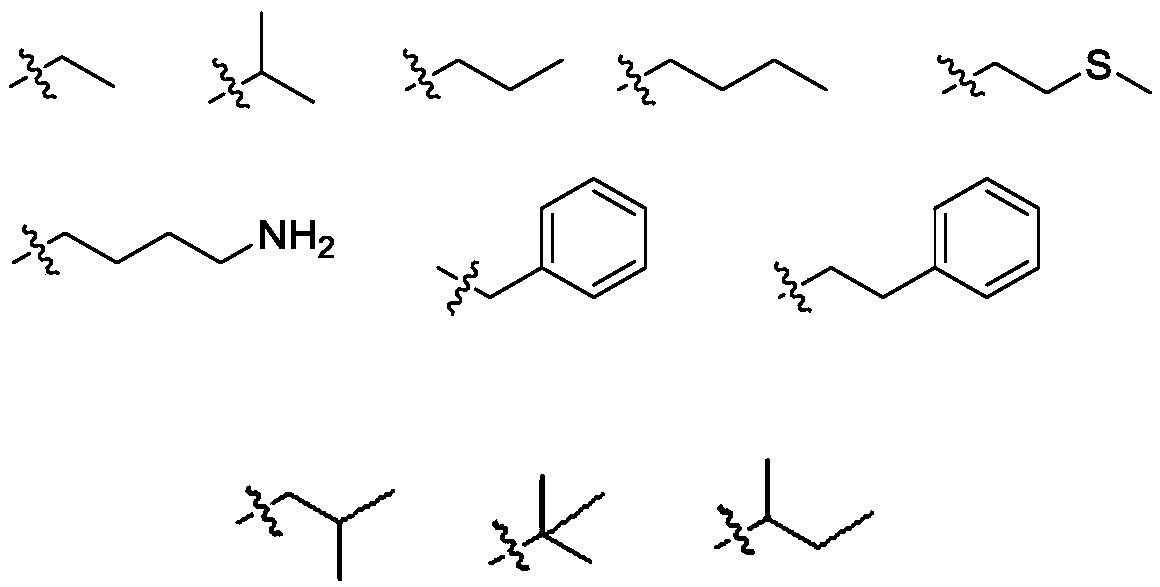
11, 20-dicarbonyl Jiyuan rubescensin a and L-amino acid-14-ester trifluoroacetate
ActiveCN110229168ASimple manufacturing methodNo allergiesOrganic chemistryAntineoplastic agentsWilms' tumorLeukemia
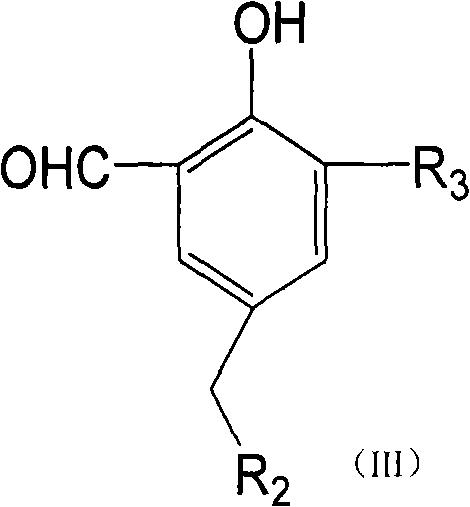
Belonging to the field of pharmaceutical compounds, the invention relates to 11, 20-dicarbonyl Jiyuan rubescensin a and L-amino acid-14-ester trifluoroacetate thereof, and a preparation method and usethereof. The preparation method includes: adopting Jiyuan rubescensin a as the raw material and performing oxidation with a Jones reagent, thus obtaining 11, 20-dicarbonyl Jiyuan rubescensin a, the structure of which is shown as the specification; then carrying out 14-position esterification reaction with N-BOC-L-amino acid to obtain N-BOC-L-amino acid-11, 20-dicarbonyl Jiyuan rubescensin a ester(III), then removing BOC protective group in trifluoroacetic acid, and then conducting salt forming to obtain L-amino acid-14-(11, 20-dicarbonyl Jiyuan rubescensin a)ester trifluoroacetate, which hasthe following general formula. The compound has good stability and anti-tumor activity, and provides the basis for screening antitumor drugs against esophageal cancer, gastric cancer, primary liver cancer, pancreatic cancer, cardiac carcinoma, colorectal cancer, bladder cancer, breast cancer, acute myelogenous leukemia and the like.
Owner:ZHENGZHOU UNIV
Preparation method and application of quaternization modified amino silicon oil softener
ActiveCN105755837AHigh epoxy valueThe promotion effect is obviousGrip property fibresVegetal fibresChemical industryTrifluoroacetic acid
The invention discloses a preparation method and application of a quaternization modified amino silicon oil softener. The preparation method comprises the following steps: I, adding trifluoroacetic acid into epoxy chloropropane, stirring, and sealing for reaction so as to obtain an upper-layer transparent solution; II, dissolving amino silicon oil into a propyl acetate solution, gradually dropping the upper-layer transparent solution prepared in the step I, stirring, and heating for reaction, thereby obtaining the quaternization modified amino silicon oil softener. The quaternization modified amino silicon oil softener prepared by using the preparation method can be applied to the softness finishing process of pure cotton textile. The invention further discloses a finishing process and belongs to the technical field of the spinning chemical industry. The preparation method disclosed by the invention is simple and convenient and easy to operate, the raw materials are green and environmentally friendly, the process is green, low in energy consumption and low in pollution, the pure cotton textile treated by using the prepared quaternization modified amino silicon oil softener is good in softness, the whiteness and the hydrophilia of the pure cotton textile are slightly affected, moreover later finishing processes of the pure cotton textile are also slightly affected, and thus relatively high market popularization values can be achieved.
Owner:江西硅博化工有限公司
Carboxylic acid betaine zwitterionic composite antibacterial functional coating material and preparation method and application thereof
ActiveCN110724426ASimple processFlexible control of the ratioAntifouling/underwater paintsPaints with biocidesQuaternary ammonium cationMedical equipment
The invention discloses a carboxylic acid betaine zwitterionic composite antibacterial functional coating material and a preparation method and application thereof. The preparation method comprises the following steps: compounding a tert-butyl-protected carboxylic acid betaine polymer containing a quaternary ammonium cation group, an alkyl chain and a catechol group at its side group with metal ions having an antibacterial function to prepare a polymer-metal ion coating liquid; treating different substrates in a coating manner and volatilizing a solution; and after leveling and drying of a coating, treating the coating with a trifluoroacetic acid solution, and performing drying to obtain the carboxylic acid betaine zwitterionic composite antibacterial functional coating diversified in construction manners and good in adaptability to various substrates. The preparation method disclosed by the invention is simple in process, mild in conditions, and friendly to environment; in the preparation process, the proportion of each functional group can be flexibly regulated and controlled; and the prepared coating material is excellent in mechanical property, good in stability, long-acting inbacterial adhesion resistance and capable of regulating and controlling cell adhesion behaviors, and has potential application value in the aspects of bioengineering, medical equipment, diagnosis andtreatment instruments and implants.
Owner:JIANGNAN UNIV
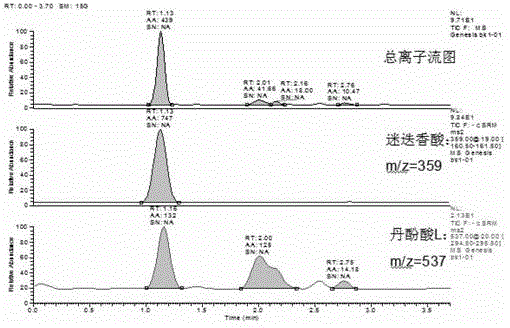
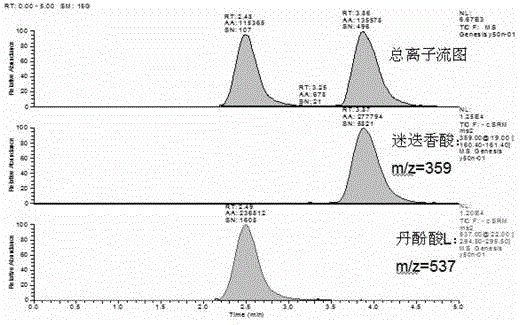
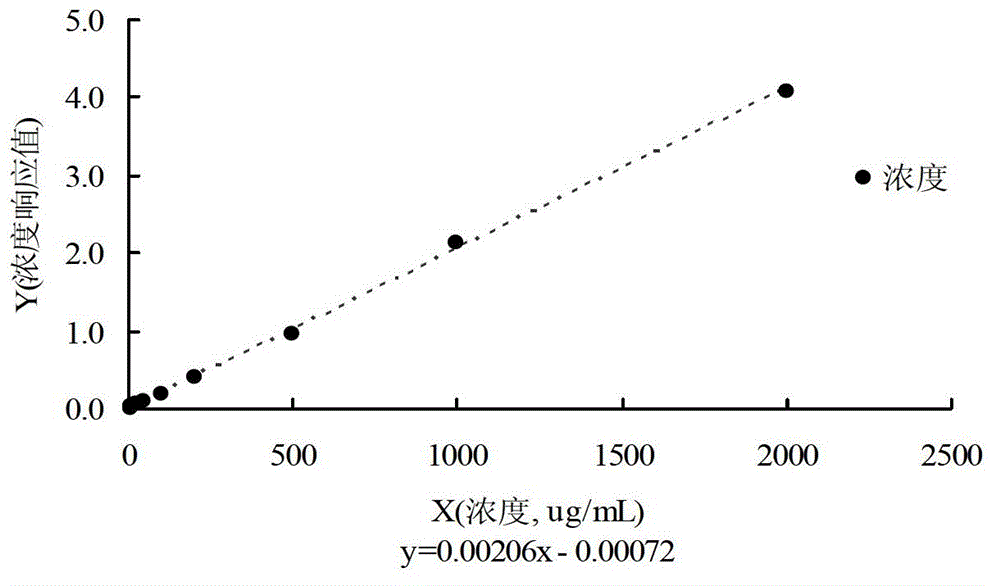
Method for determining salvianolic acid L in blood plasma
The invention relates to a method for determining content of medicine in blood plasma, which comprises the following steps: 1)treating a sample; taking the sample of blood plasma, adding a proper amount of an acid solution and an internal standard solution, mixing; adding an organic solvent for mixing and then extracting; centrifuging the above mixed liquor and taking an organic solvent layer, blowing under water bath, then redissolving by a liquid phase mobile phase and redissolving, taking an supernatant for sample introduction and determining; 2)separating liquid phase: taking an anti-phase silica gel chromatographic column as a separating medium of a liquid phase part of a liquid chromatograph / mass spectrometer, performing isocratic elution by a mobile phase with a fixed ratio after sample introduction, preliminarily separating an interferent, an object to be detected, and the internal standard; wherein a mobile phase A is selected from a formic acid aqueous solution, an acetate aqueous solution, a trifluoroacetic acid aqueous solution or an ammonium acetate aqueous solution, a mobile phase B is selected from acetonitrile or methanol, and the phase A accounts for 30-90% of whole mobile phase; and 3)detecting by mass spectrum: according to set ionizationoun condition, the mass spectrum part of the liquid chromatograph / mass spectrometer employs a SRM scan function for detect the salvianolic acid L in blood plasma and the content of the internal standard.
Owner:TIANJIN TASLY PHARMA CO LTD
MOFs supported catalyst, preparation method thereof, and application in olefin hydrosilylation reaction
ActiveCN104907096AEfficient separationNo residueSilicon organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsOrganometallic catalysisBenzaldehyde
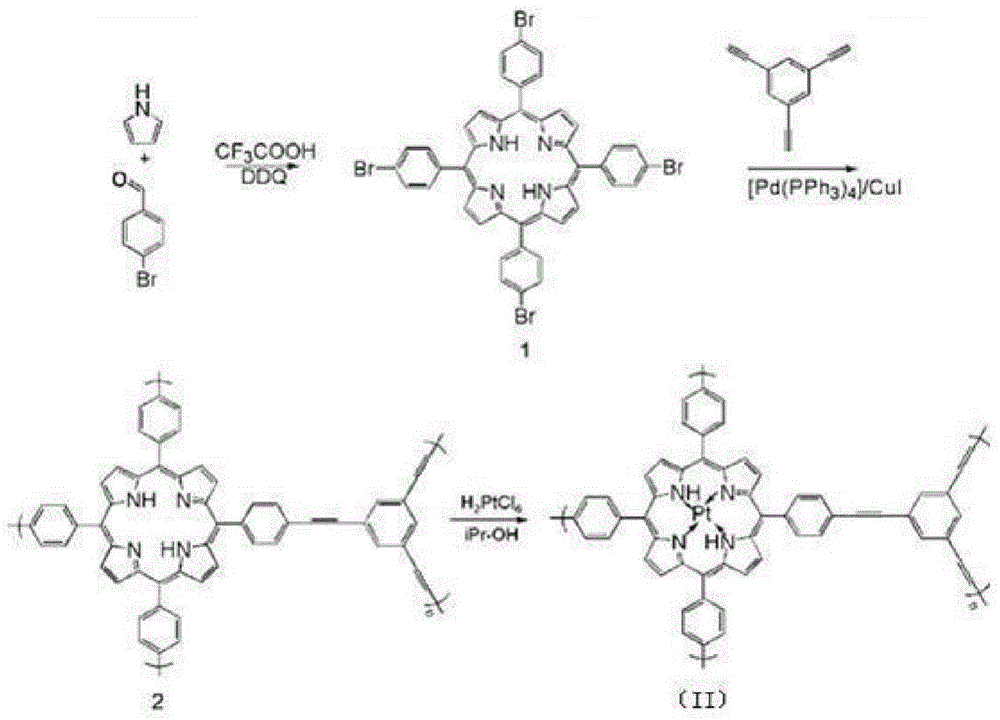
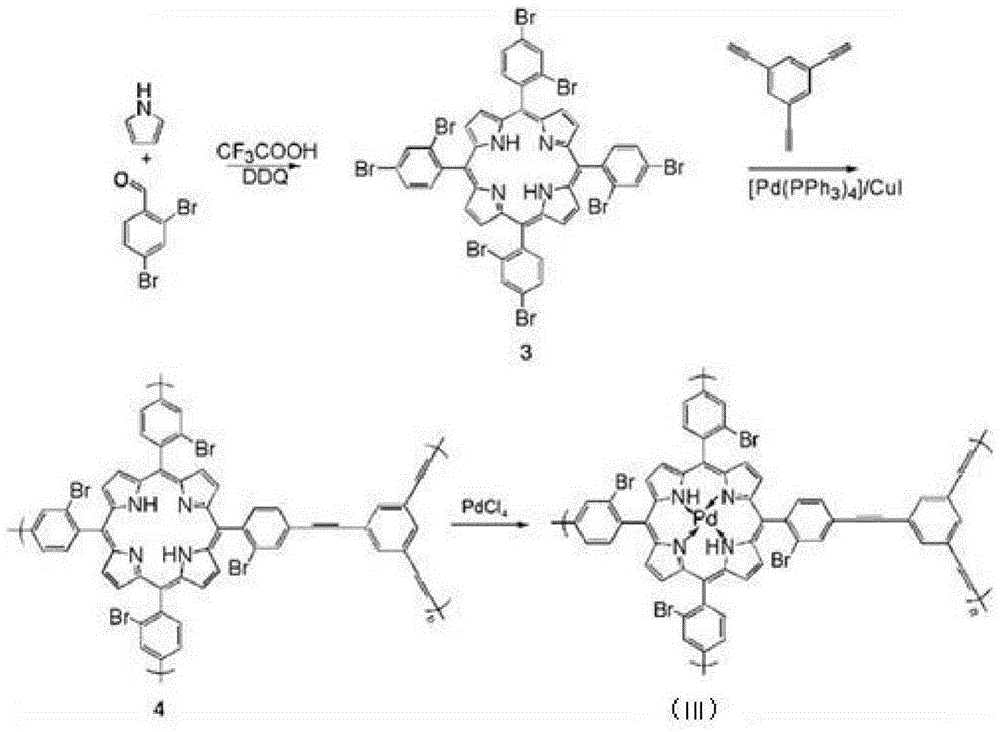
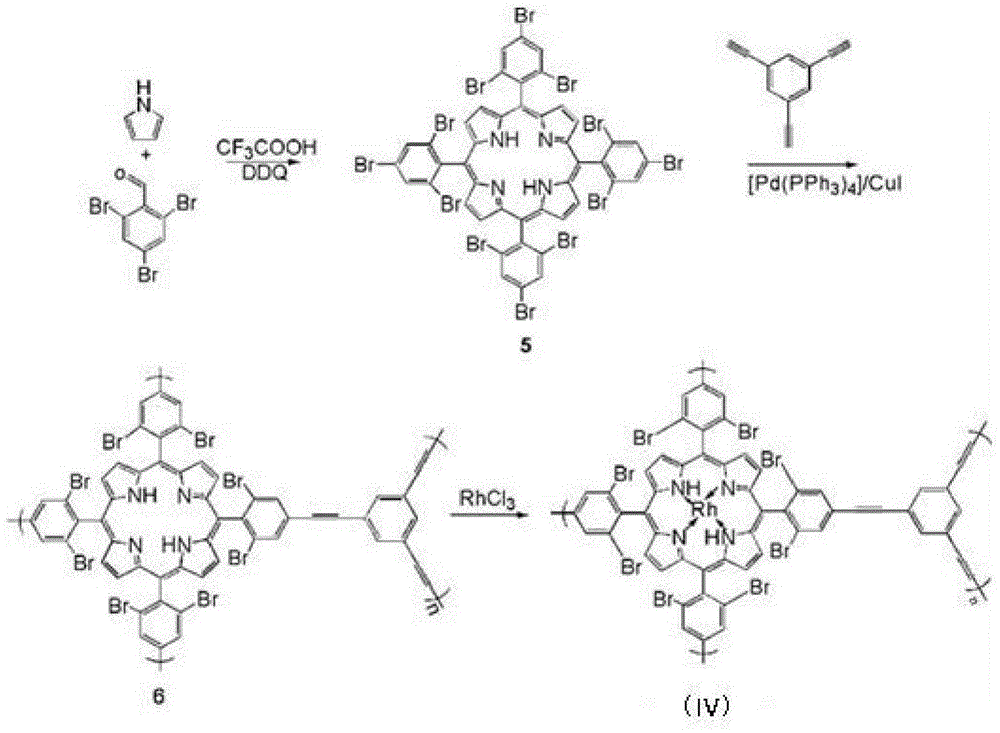
The invention belongs to the technical field of organic metal catalysis, and concretely relates to an MOFs supported catalyst, a preparation method thereof, and an application in an olefin hydrosilylation reaction. The catalyst has a structure represented by formula (I); and in the formula (I), M is metallic platinum, rhodium, palladium or ruthenium, and R1 and R2 can be H or Br, and can be same to or different from each other. The preparation method of the catalyst comprises the following steps: adding pyrrole, bromine-substituted benzaldehyde and a solvent into a reactor, adding trifluoroacetic acid and DDQ, and reacting to obtain an organic framework monomer; reacting the organic framework monomer with 1,3,5-triethynylbenzene to obtain an organic framework polymer; and reacting a homogeneous solution of the metallic platinum, rhodium, palladium or ruthenium with the organic framework polymer to obtain the catalyst. The MOFs supported catalyst can efficiently catalyze the hydrosilylation reaction of hydrogen-containing silane and olefin, can be recycled through a simple technology, and can effectively improve the utilization rate of precious metals.
Owner:GUANGZHOU TINCI MATERIALS TECH
MALDI-TOF-MS (Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry) detection method of fluoroquinolone medicines in milk
InactiveCN103743847AAdapt to the requirements of quick inspection and quick releaseEfficient detectionComponent separationMolecular sieveFluoro quinolones
The invention discloses an MALDI-TOF-MS (Matrix-Assisted Laser Desorption / Ionization Time-of-Flight Mass Spectrometry) detection method of fluoroquinolone medicines in milk. The method comprises the following steps: sieving 8-hydroxyquinoline by a functional mesoporous molecular sieve to obtain SBA (Santa Barbara Amorphous material)-15-8-hydroxyquinoline; diluting milk by trifluoroacetic acid to obtain a sample target object; dispersing the SBA-15-8-hydroxyquinoline in ethanol liquor and carrying out ultrasonic treatment to obtain suspension liquid; adding the suspension liquid into the sample target object, and naturally drying at room temperature to obtain an MALDI matrix thin layer; placing milk to be detected on the MALDI matrix thin layer, carrying out MALDI-TOF-MS analysis after a solvent is naturally evaporated, and dropping a sample and the matrix on a target plate in a mixed manner, and detecting after the target plate directly enters into the MALDI-TOF-MS, wherein a fuzzy pre-treatment process is canceled. When the residual amount of the fluoroquinolone medicines in milk exceeds 0.05mg / kg, 18 of 25 fluoroquinolone medicines in milk can be effectively detected, so that the detection efficiency is greatly improved so as to meet the demand on quick test and quick release of import and export products.
Owner:张金玲
Method for producing chlorfenapyr raw material pesticide
The invention discloses a method for producing a chlorfenapyr raw material pesticide. The method comprises the following steps of: adding chlorobenzol glycine and trifluoroacetic acid which serve as raw materials and 4-dimethylamino pyridine serving as a catalyst into an acetonitrile solvent, stirring uniformly, and dripping a phosphorus trichloride solution to perform acylation reaction; after the reaction is finished, adding 2-chloro acrylonitrile serving as a raw material and dimethyl formamide (DMF) serving as a cosolvent, and stirring for dissolving; dripping a triethylamine solution to perform cyclization reaction, and dripping bromine into the reaction solution; after bromination reaction is finished, and removing the solvent and the cosolvent; adding the residual substance into an alcohol solvent for dissolving, and cooling to precipitate an intermediate; adding the intermediate and chloromethyl ethyl ether into ethyl acetate serving as a solvent, stirring for dissolving, dripping triethylamine to perform condensation reaction, removing the solvent and the ethyl acetate, and hydrolyzing to obtain the chlorfenapyr raw material pesticide. In the method, the acylation reaction and the cyclization reaction are completed in one step, so a process is simplified, energy consumption is reduced, a production process is energy-saving and environment-friendly, and the purity and yield of products are improved.
Owner:山东亿嘉农化有限公司
Formation method of lithium ion battery
InactiveCN111370792AInhibition of IntercalationImprove stabilityFinal product manufactureSecondary cells charging/dischargingElectrolytic agentPerfluoroacetic Acid
The invention provides a formation method of a lithium ion battery. A negative electrode active substance of the lithium ion battery is a graphite material; the electrolyte of the lithium ion batterycomprises lithium salt, an organic solvent and an additive, the organic solvent comprises ethylene carbonate, dimethyl carbonate and propylene carbonate, the additive comprises 1, 2-trifluoroacetic acid ethyl ethane, dimethyl sulfoxide and anisole, and the formation method comprises: injecting a first electrolyte accounting for 45-50 vol% of the total amount of the electrolyte, wherein the organicsolvent of the first electrolyte is ethylene carbonate, the additive is 1, 2-trifluoroacetic acid ethyl ethane, and the content of the 1, 2-trifluoroacetic acid ethyl ethane is 8-12% by volume; the organic solvent of the second electrolyte comprises propylene carbonate and dimethyl carbonate, the additives are dimethyl sulfoxide and anisole, and the content of dimethyl sulfoxide is 3.6-4.0% by volume and the content of the anisole is 0.15%-0.3% by volume; and performing formation to obtain the battery.
Owner:朱虎
Method for measuring optical purity of R-alogliptin benzoate
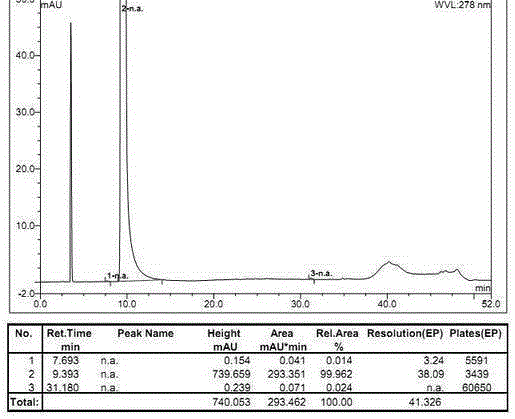
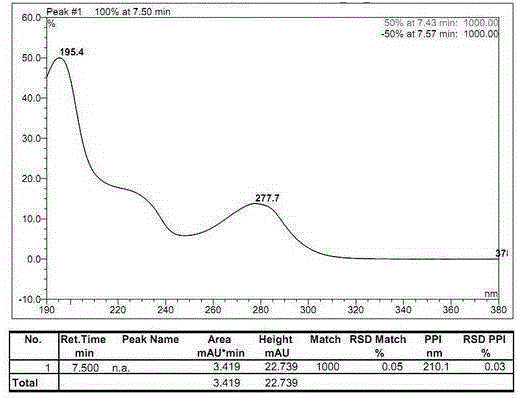
The invention relates to a method for measuring the optical purity of R-alogliptin benzoate. The method is characterized in that a chromatographic system is a high performance liquid chromatograph; a chromatographic column filler is amylose-tris(3,5-dimethylphenyl carbamate); the flow velocity is 0.8 ml / min; the column temperature is 25 DEG C; the measurement wavelength is 278 nm; the sample introduction amount is 20 [mu]l; the chromatographic mobile phases are n-hexane and an ethanol solution which contains 0.2% trifluoroacetic acid and 0.1% diethyl amine; the ratio of n-hexane to the ethanol solution is 80:20; sample introduction is conducted on an R,S-alogliptin benzoate reference solution and an S-alogliptin benzoate reference solution respectively for system suitability analysis at first, and then the optical purity of an R-alogliptin benzoate test solution is measured and the optical purity of an R-alogliptin benzoate sample is calculated according to an area normalization method.
Owner:DISHA PHARMA GRP +1
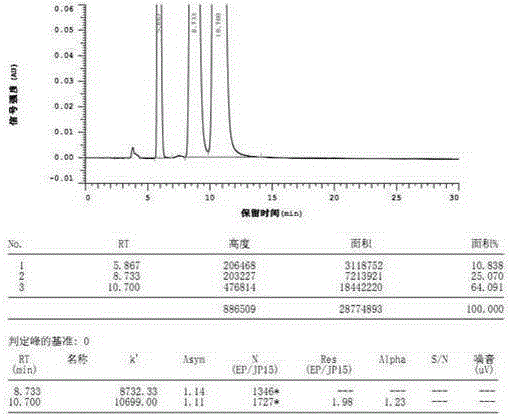
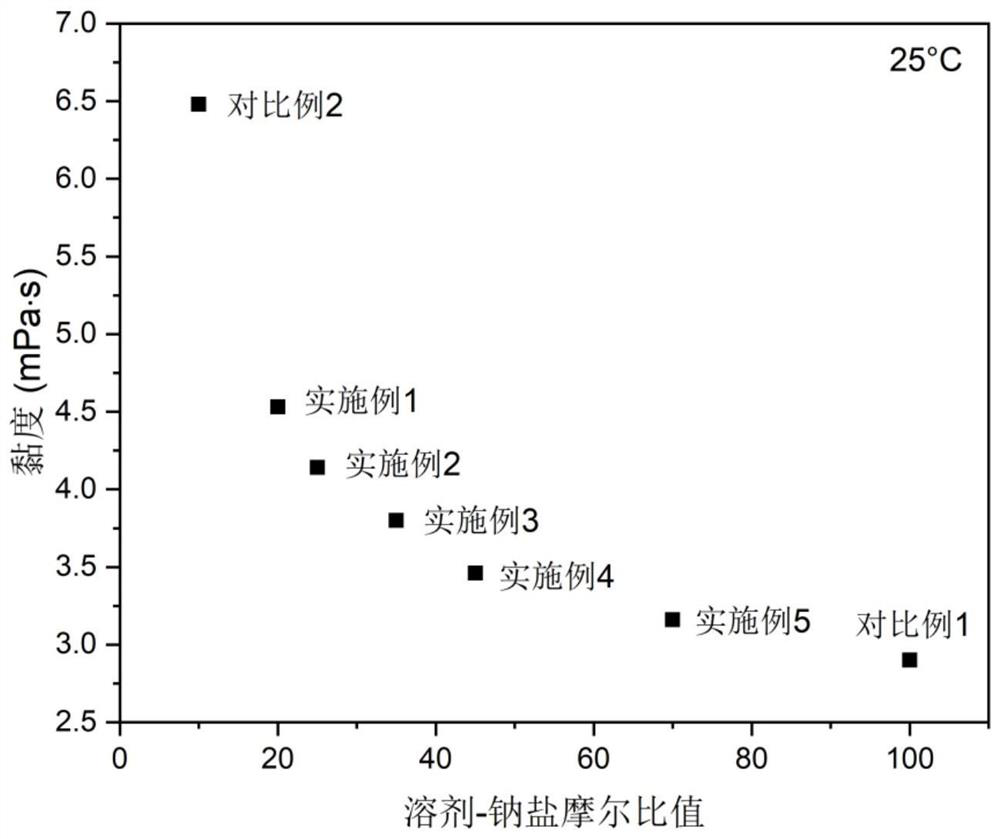
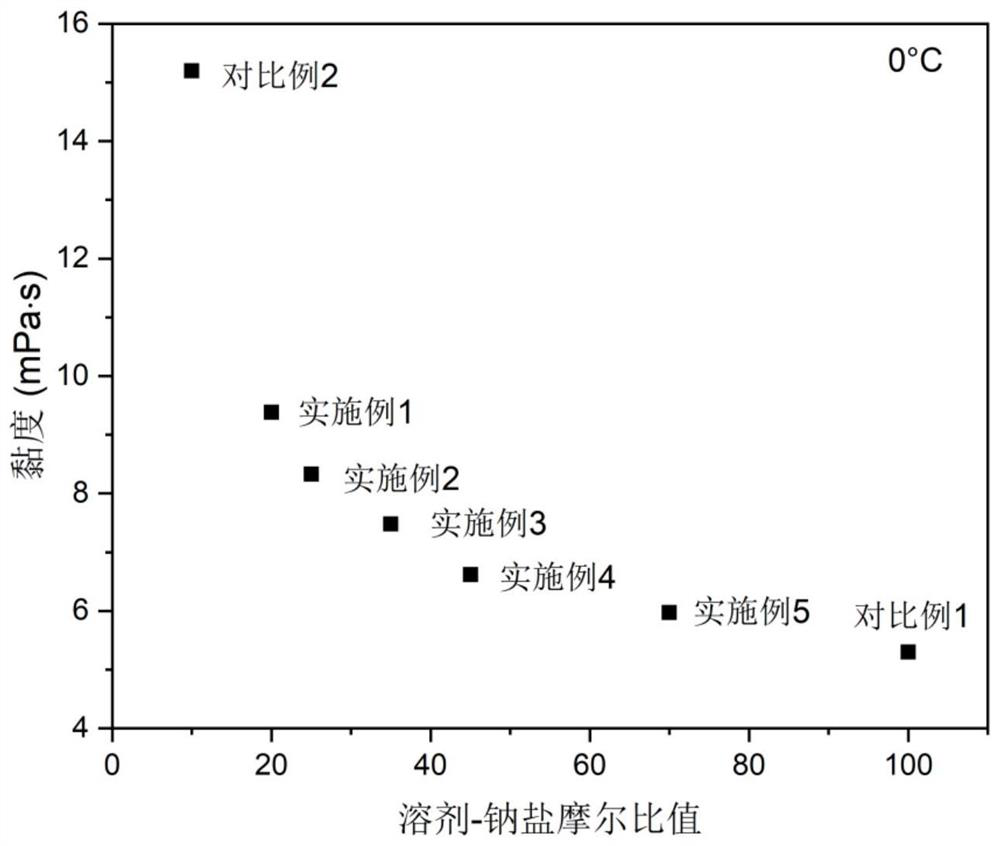
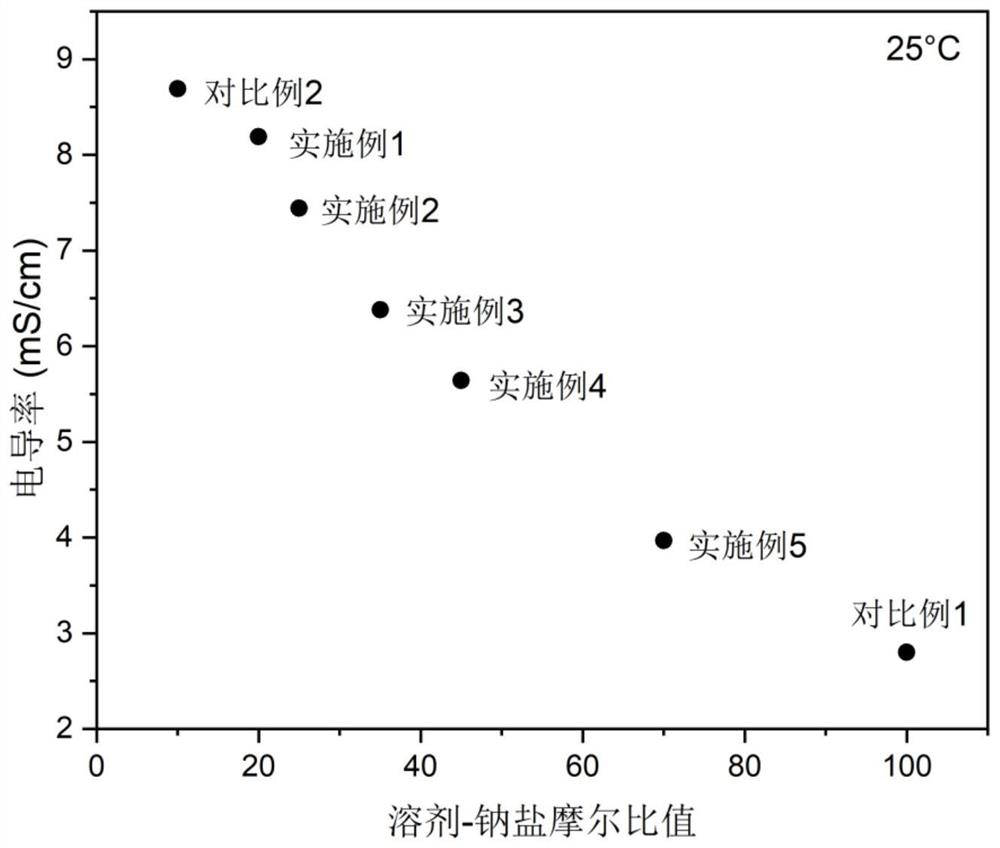
Electrolyte with high solvent-sodium salt ratio and sodium ion battery
PendingCN113299976AReduce viscosityImprove wettabilitySecondary cellsElectrolytic agentSodium tetrafluoroborate
The invention discloses an electrolyte with a high solvent-sodium salt ratio and a sodium ion battery. The electrolyte comprises sodium salt, a solvent and an additive, wherein the molar ratio of the solvent to the sodium salt is (20: 1)-(70: 1), and the addition amount of the additive accounts for 0.5%-5% of the mass of the electrolyte; the sodium salt comprises one or more of sodium perchlorate (NaClO4), sodium tetrafluoroborate (NaBF4), sodium hexafluorophosphate (NaPF6), sodium hexafluoroarsenate (NaAsF6), sodium trifluoroacetate (CF3COONa), sodium tetraphenylborate (NaB(C6H5)4), sodium trifluoromethanesulfonate (NaSO3CF3), sodium bis (fluorosulfonyl) imide (Na[(FSO2)2N]) or sodium bis (trifluoromethanesulfonyl) imide (Na[(CF3SO2)2N]).
Owner:INST OF PHYSICS - CHINESE ACAD OF SCI
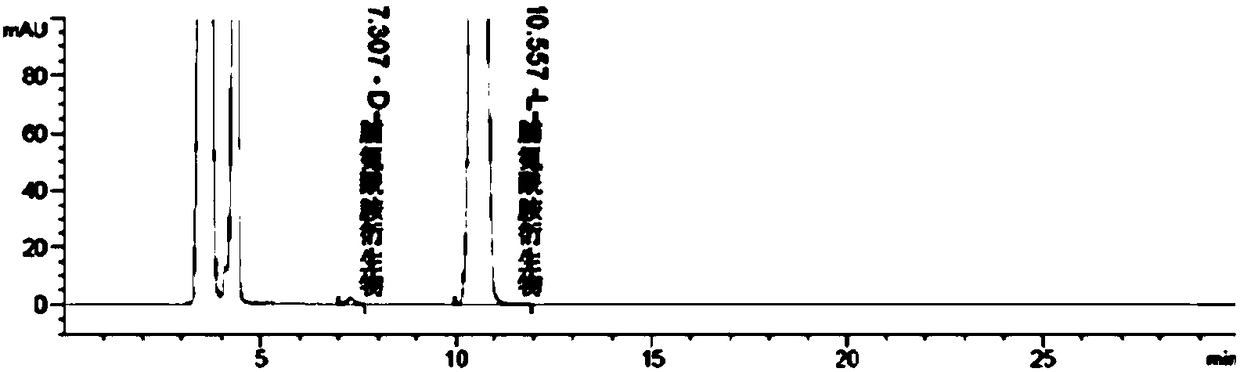
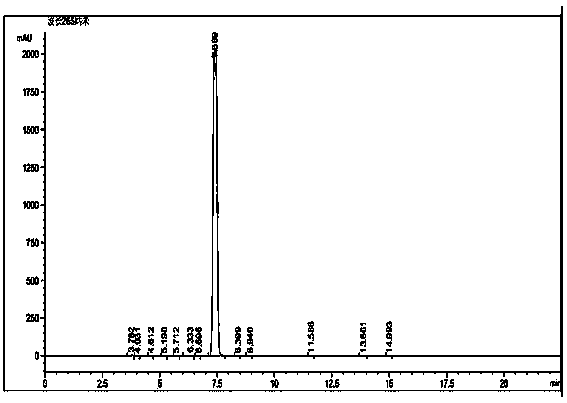
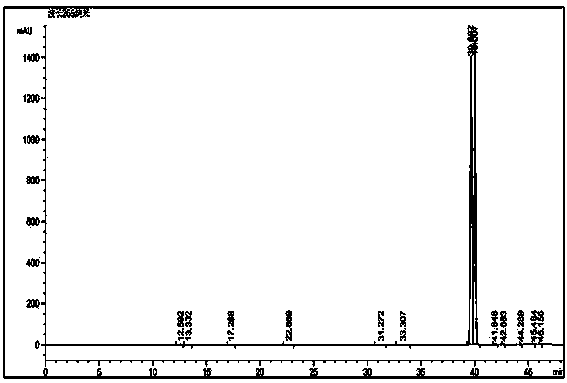
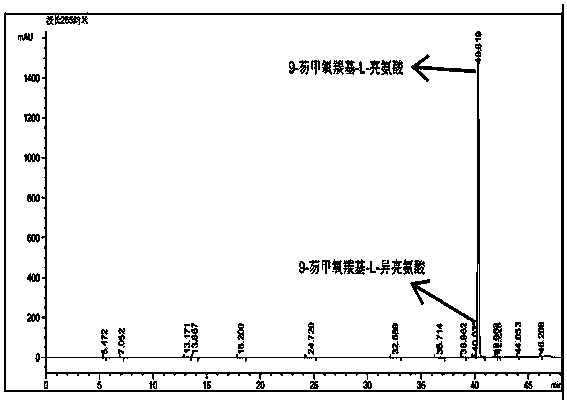
Method for reversed phase separation of derived leucine and isoleucine
The invention relates to a method for reversed phase separation of amino acids containing impurities, and mainly solves the technical problems of high cost and high device requirements of present methods for quantitatively determining 18 composite amino acids through pre-column derivatization. The method includes the following steps: (1) preparing a mobile phase A and a mobile phase B needed by analysis, and carrying out ultrasonic mixing, wherein the mobile phase A is an aqueous solution of trifluoroacetic acid, and the mobile phase B is an acetonitrile solution of trifluoroacetic acid; (2) introducing the prepared mobile phases into a chromatographic system, inspecting all systems of a high performance liquid chromatograph to ensure that the chromatograph is normal, setting chromatographic conditions, and balancing a chromatographic column according to the gradient the initial concentrations; (3) dissolving N-(9-fluorenylmethyloxycarbonyl)-L-leucine, N-(9-fluorenylmethyloxycarbonyl)-L-isoleucine and a mixture of the above two substances in acetonitrile, and filtering to obtain single sample liquids and a mixed liquid for later use; and (4) respectively sucking the mixed liquid and the two sample liquids into the chromatographic system after chromatographic system balancing, acquiring chromatographic data, and carrying out area normalization method integration on the obtained chromatograms.
Owner:CS BIO SHANGHAI
High-temperature superconducting nanometer composite film and method for preparing same
ActiveCN101747031AIncrease the critical current densitySuperconductors/hyperconductorsSuperconductor devicesFluoroacetic acidComposite film
The invention provides a method for preparing a high-temperature superconducting nanometer composite film, which comprises the following steps: preparing a precursor liquid, mixing Y(CH3COO)3, Ba(CH3COO) and Cu(CH3COO) in a molar ratio of 1:2:3 and dissolving the mixture in 20 to 30 mol percent aqueous solution of trifluoroacetic acid; uniformly stirring the solution and drying a solvent by distillation to obtain gel; then adding methanol in the gel, uniformly stirring the mixture, drying the solvent by distillation to obtain gel; adding methanol and zirconium(IV) acetylacetonat in an amount of 5 to 8 mol percent based on the total ion concentration of three metals to prepare precursor liquid, and the coating the precursor liquid on a monocrystal oxidate substrate with a two-inch diameter by the method of rotatable coating or lifting and pulling. The coated film is subjected to low temperature thermal treatment at 400 and 410 DEG C first to decompose trifluoroacetate, and finally the YBCO film containing nanometer barium zirconate is formed through the thermal treatment at the high temperature of between 800 and 850 DEG C and the annealing process at the temperature of between 490 and 510 DEG C.
Owner:GRIMAT ENG INST CO LTD
Preparation method and application of nitrogen-doped graphene-based carbon aerogel microspheres
InactiveCN110817871AClean appearanceNo leakageCarbon compoundsHybrid capacitor electrodesElectrolytic agentDoped graphene
The invention relates to the technical fields of gel microsphere preparation and applications, and in particular relates to a preparation method and application of nitrogen-doped graphene-based carbonaerogel microspheres. The method includes the following steps: S1, mixing resorcinol, formaldehyde, catalyst anhydrous sodium carbonate, graphene oxide, melamine and deionized water, performing a reaction to obtain a reaction solution; S2, adding the reaction solution into a surfactant, and performing stirring to obtain a uniformly dispersed red-brown emulsion; S3, performing a sol-gel reaction on the red-brown emulsion, performing aging by using an acetone solution containing trifluoroacetic acid, performing soaking by using acetone, and performing normal-pressure drying to obtain an organicaerogel; S4, performing carbonization to obtain graphene-based carbon aerogel microspheres; S5, performing KOH activation to obtain a spherical carbon aerogel; and S6, performing CO2 activation. Thecarbon aerogel microspheres prepared by the method have a moderate particle size, a higher bulk density and higher porosity, a high effective specific surface area, excellent electrical conductivity,and good wettability with an electrolyte.
Owner:GUIZHOU MEILING POWER SUPPLY CO LTD
Dithieno[2,3-b:2',3'-d]thiophene preparation methods
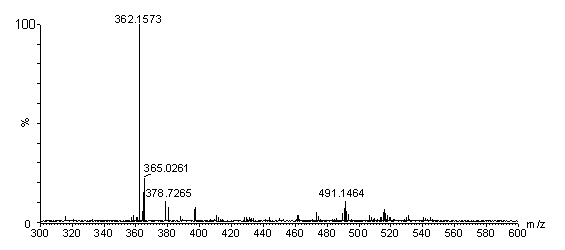
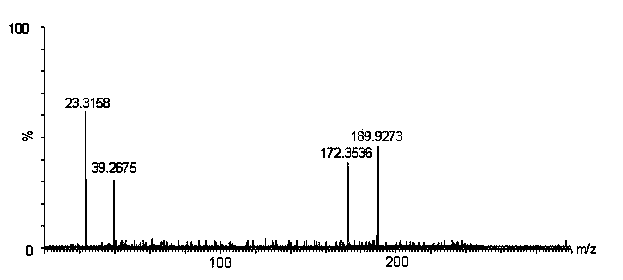
The present invention discloses two dithieno[2,3-b:2',3'-d]thiophene preparation methods. The method 1 comprises: adopting 2,3'-dibromo-3,2'-bithiophene as a raw material, and carrying out t-BuLi double bromine lithium exchange and thiolizing reagent (PhSO2)2S ring closure to prepare the dithieno[2,3-b:2',3'-d]thiophene; or adopting 5,5'-bis(trimethylsilyl)-2,3'-dibromo-3,2'-bithiophene as a raw material, carrying out double bromine lithium exchange through n-BuLi, and thiolizing reagent (PhSO2)2S ring closure, and removing trimethylsilyl through trifluoroacetic acid to prepare the dithieno[2,3-b:2',3'-d]thiophene. The method 2 comprises: adopting 3-bromo-2,3'-bithiophene as a raw material, carrying out n-BuLi bromine lithium exchange while carrying out selective competition of protons, and carrying out thiolizing reagent (PhSO2)2S ring closure to prepare the dithieno[2,3-b:2',3'-d]thiophene; or adopting 3-bromo-5-(trimethylsilyl)-2,3'-bithiophene as a raw material, carrying out n-BuLi bromine lithium exchange while carrying out selective competition of protons, carrying out thiolizing reagent (PhSO2)2S ring closure and removing trimethylsilyl through trifluoroacetic acid to prepare the dithieno[2,3-b:2',3'-d]thiophene. According to the present invention, the synthesis process has operability, reaction conditions relate to no water, no oxygen and low temperature, and the method is suitable for laboratory scale preparation.
Owner:HENAN UNIVERSITY
Preparation method for flexible composite loaded with macro MOFs efficiently
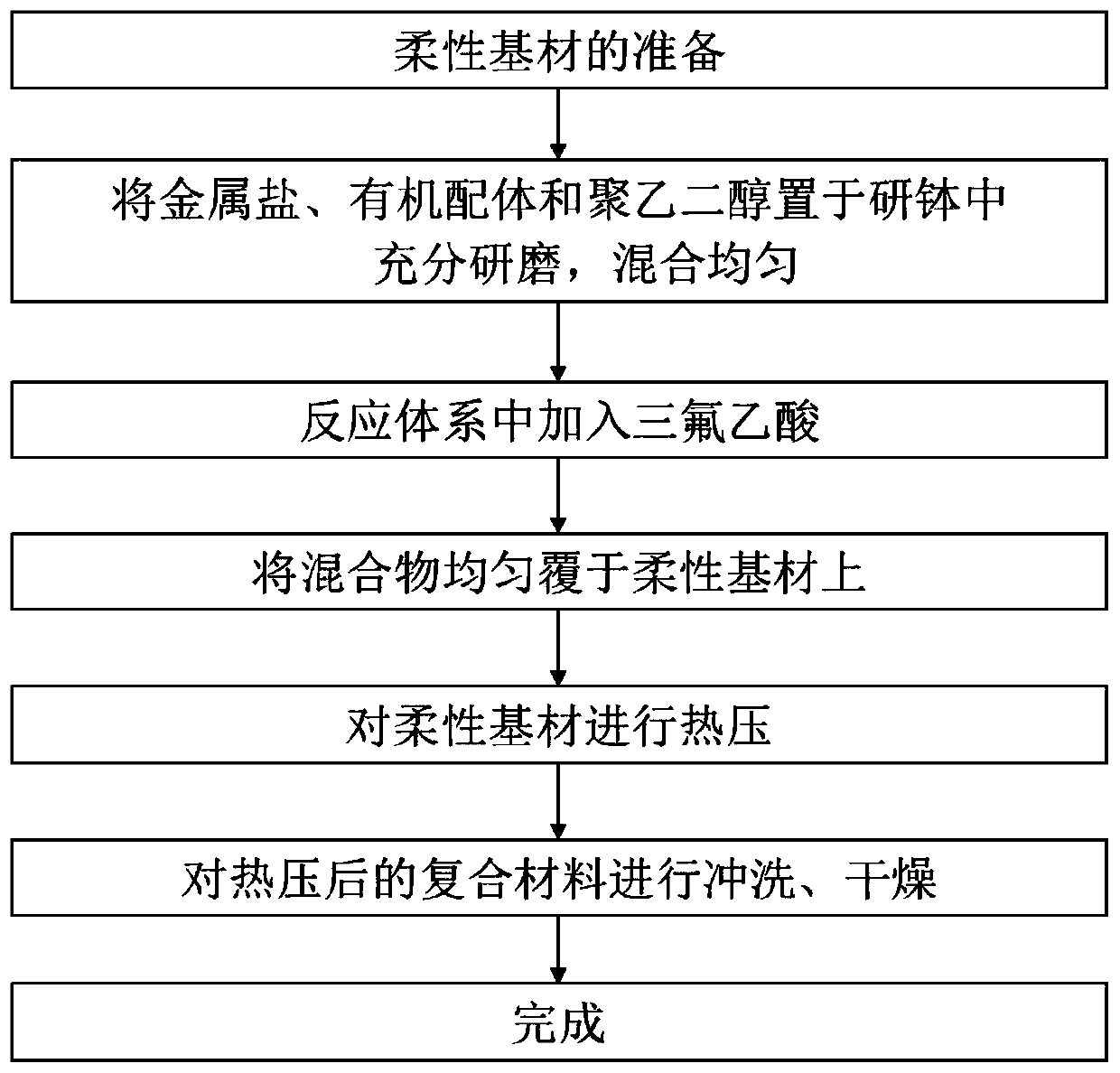
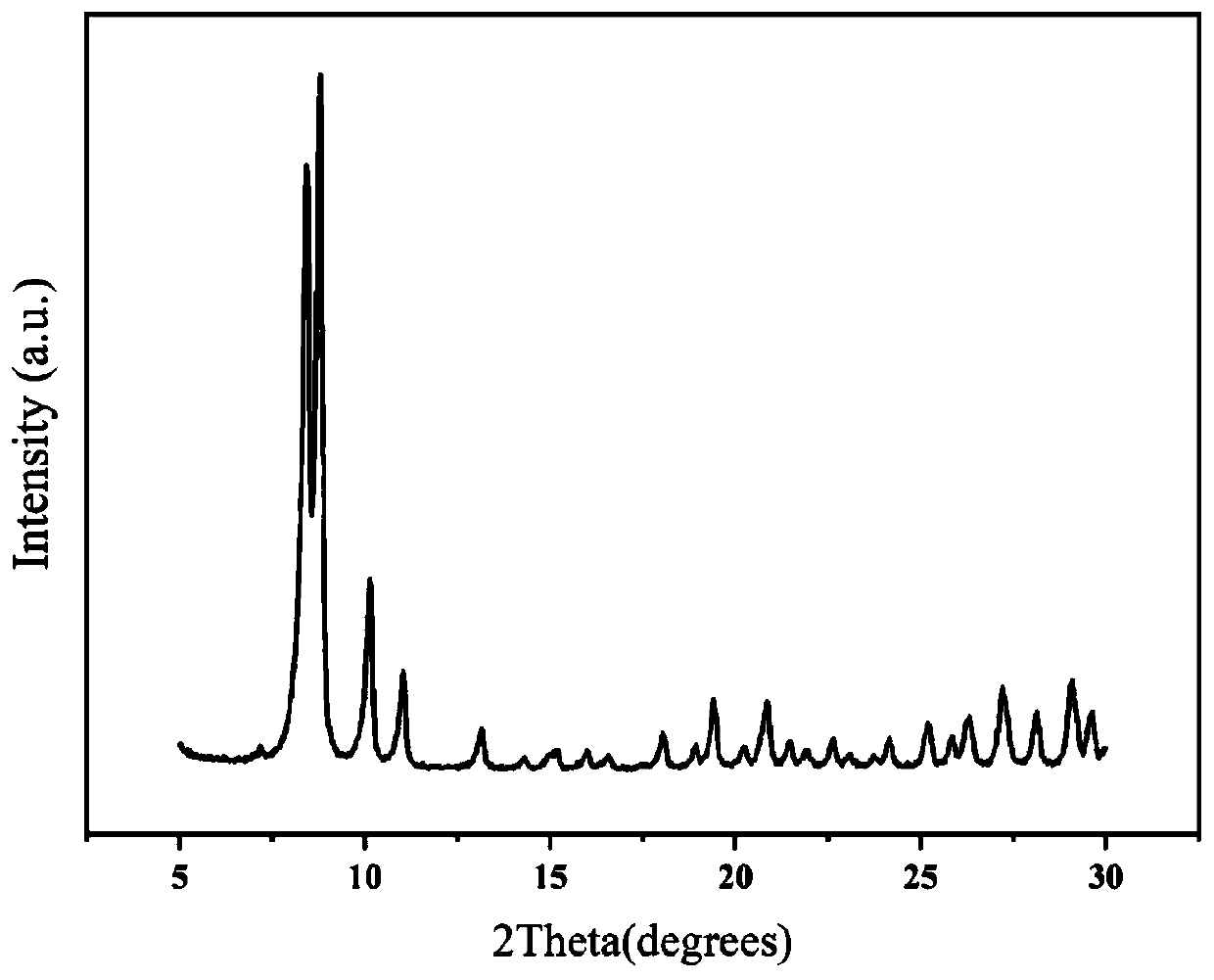
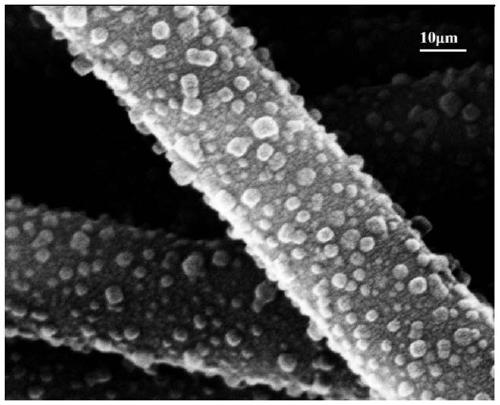
The invention provides a preparation method for a flexible composite loaded with macro MOFs efficiently, and belongs to the technical field of composites. Metal-organic frameworks (MOFs) are combinedwith nano, micro-nano or micron textiles effectively through a hot pressing method. By means of the supporting function of a flexible substrate for the MOFs, the agglomeration problem of the MOFs dueto powder properties, and the problem of power application restriction are reduced; through introduction of trifluoroacetic acid into a system, more MOFs with metal cluster defects and ligand defectsare manufactured in the self-assembly process of the MOFs, and meanwhile more active groups are exposed to the surfaces of the flexible composite; and the combination between the MOFs and the flexiblecomposite is improved, so that the MOFs are combined with the flexible composite more securely, and are not liable to fall out. The MOFs / flexible composite prepared with the preparation method has the characteristics of uniform fiber size, high MOFs loading capacity and excellent mechanical property of films. The preparation method has the advantages of low cost, simple process, low time consumption, good environmental friendliness, capability of macro production, and wide industrial application prospect.
Owner:UNIV OF SCI & TECH BEIJING
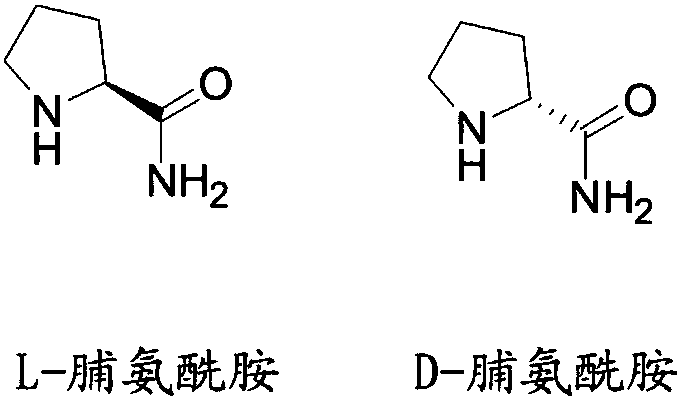
Liquid chromatographic analysis method of L-prolinamide
InactiveCN108226329ACompliant with derivatization requirementsHigh UV absorptionOrganic chemistryComponent separationTrifluoroacetic acidDerivatization
The invention provides a liquid chromatographic analysis method of L-prolinamide. The liquid chromatographic analysis method comprises the following steps: precisely measuring an L-prolinamide solution, a triethylamine ethanol solution and a derivatization reagent solution respectively, and uniformly mixing through vortex; reacting for a period of time; then adding a diethylamine ethanol solutionand uniformly mixing through the vortex; reacting for a period of time; then adding a trifluoroacetic acid ethanol solution and uniformly mixing through the vortex to obtain an L-prolinamide derivative solution; carrying out chromatographic analysis on the L-prolinamide derivative solution. The liquid chromatographic analysis method provided by the invention can be used for accurately and sensitively detecting the L-prolinamide and D-prolinamide.
Owner:ZHEJIANG TIANYU PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com




![Dithieno[2,3-b:2',3'-d]thiophene preparation methods Dithieno[2,3-b:2',3'-d]thiophene preparation methods](https://images-eureka.patsnap.com/patent_img/f5eb0a16-e8fc-4c39-bc39-51cc2ef1298c/HDA00003112265200011.PNG)
![Dithieno[2,3-b:2',3'-d]thiophene preparation methods Dithieno[2,3-b:2',3'-d]thiophene preparation methods](https://images-eureka.patsnap.com/patent_img/f5eb0a16-e8fc-4c39-bc39-51cc2ef1298c/HDA00003112265200012.PNG)
![Dithieno[2,3-b:2',3'-d]thiophene preparation methods Dithieno[2,3-b:2',3'-d]thiophene preparation methods](https://images-eureka.patsnap.com/patent_img/f5eb0a16-e8fc-4c39-bc39-51cc2ef1298c/HDA00003112265200013.PNG)