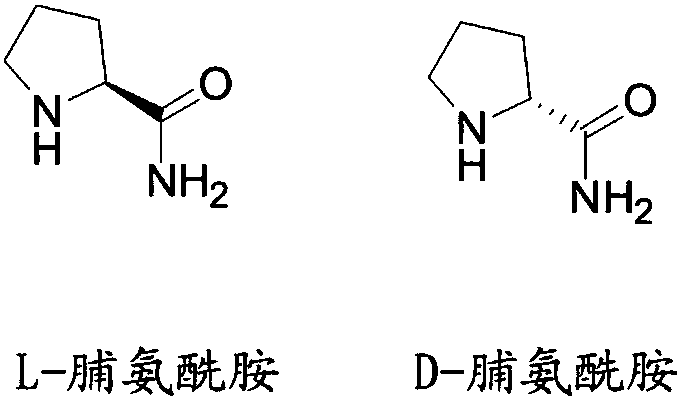
Liquid chromatographic analysis method of L-prolinamide
A liquid chromatographic analysis, prolineamide technology, applied in the direction of analysis materials, material separation, measuring devices, etc., can solve the problems of weak ultraviolet absorption, difficult sample detection, etc., and achieve the effect of high separation degree
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0038] specific implementation plan
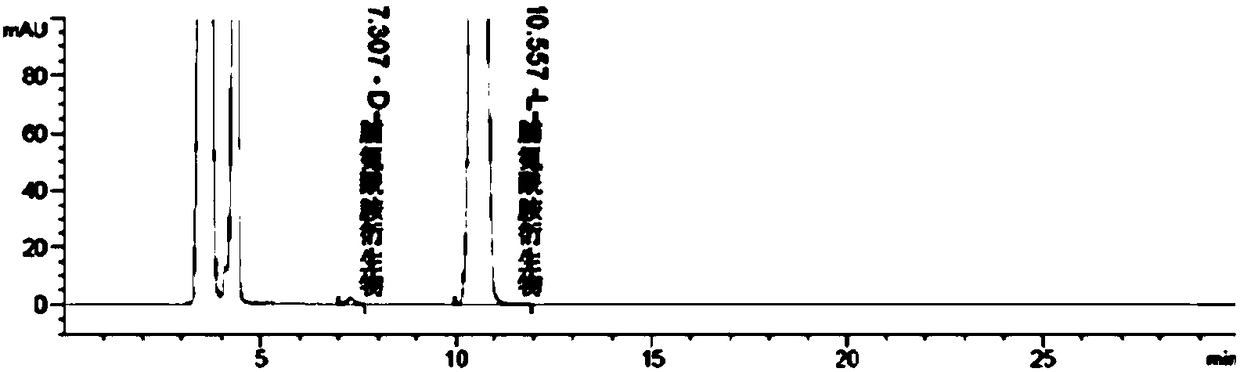
[0039] Testing equipment : High performance liquid chromatography equipped with UV detector
[0040] Chromatographic conditions :
[0041] Chromatographic column: DAICEL CHIRALPAK IC 4.6×250mm, 5um
[0042] Mobile phase: n-hexane-ethanol (40 / 60(v / v))
[0043] Detection wavelength: 262nm
[0044] Flow rate: 1.0ml / min
[0045] Column temperature: 30°C
[0046] Injection volume: 10μL
[0047] Sample tray temperature control: 15°C
[0048] Running time: 30min
[0049] Solution preparation :
[0050] Diluent: Accurately pipette 1.0ml of trifluoroacetic acid into 1000ml of ethanol and mix well.
[0051] Test solution: Accurately weigh 50mg of L-prolinamide sample in a 25ml volumetric flask, dissolve and dilute to the mark with diluent, and shake well.
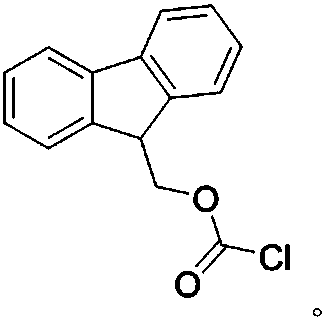
[0052] FMOC-Cl solution: Accurately weigh 15 mg of FMOC-Cl sample, dissolve it in 5 ml of acetonitrile, and mix well. (sealed, stored at 4°C, ready to use).
[0053] 1% triethyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com