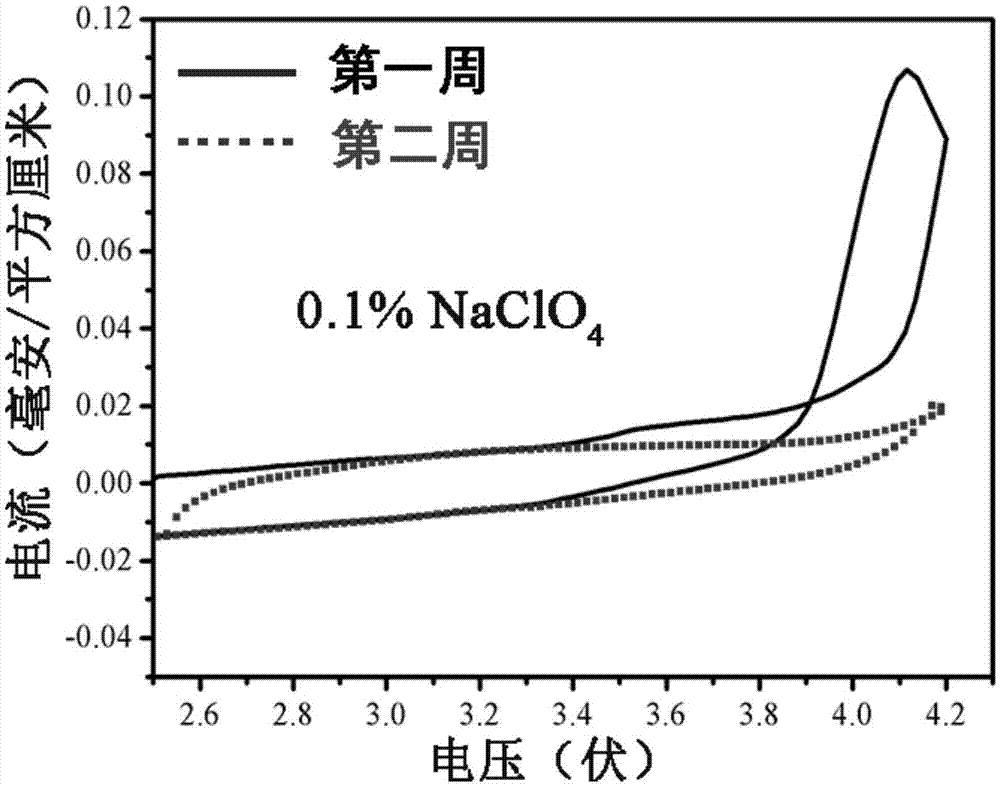
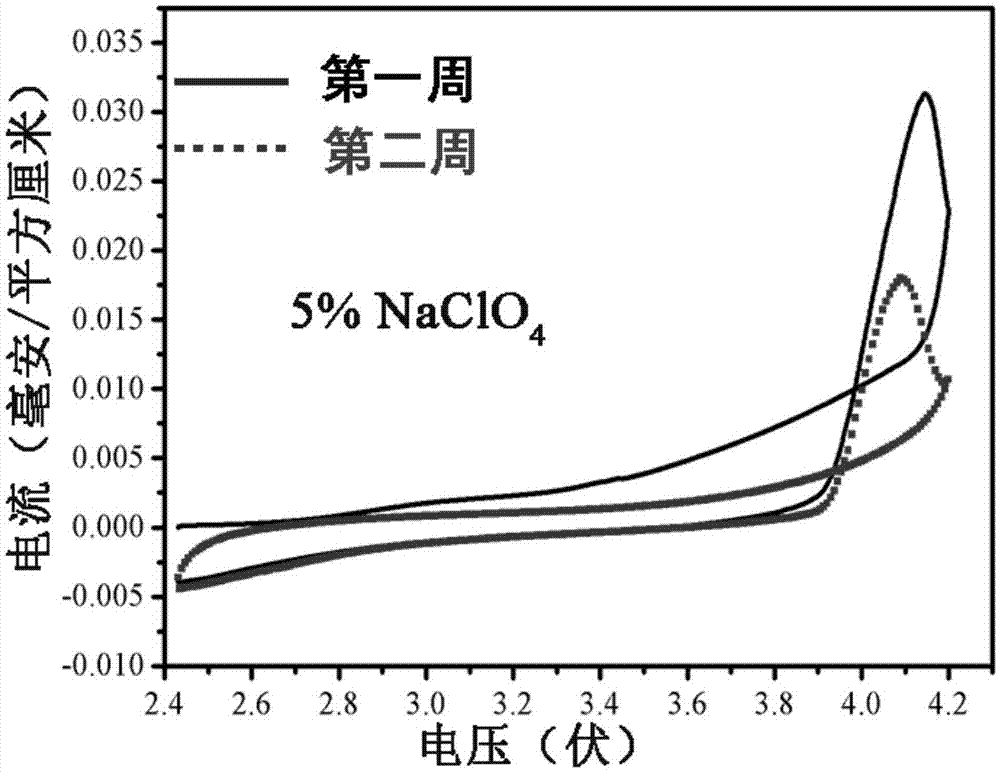
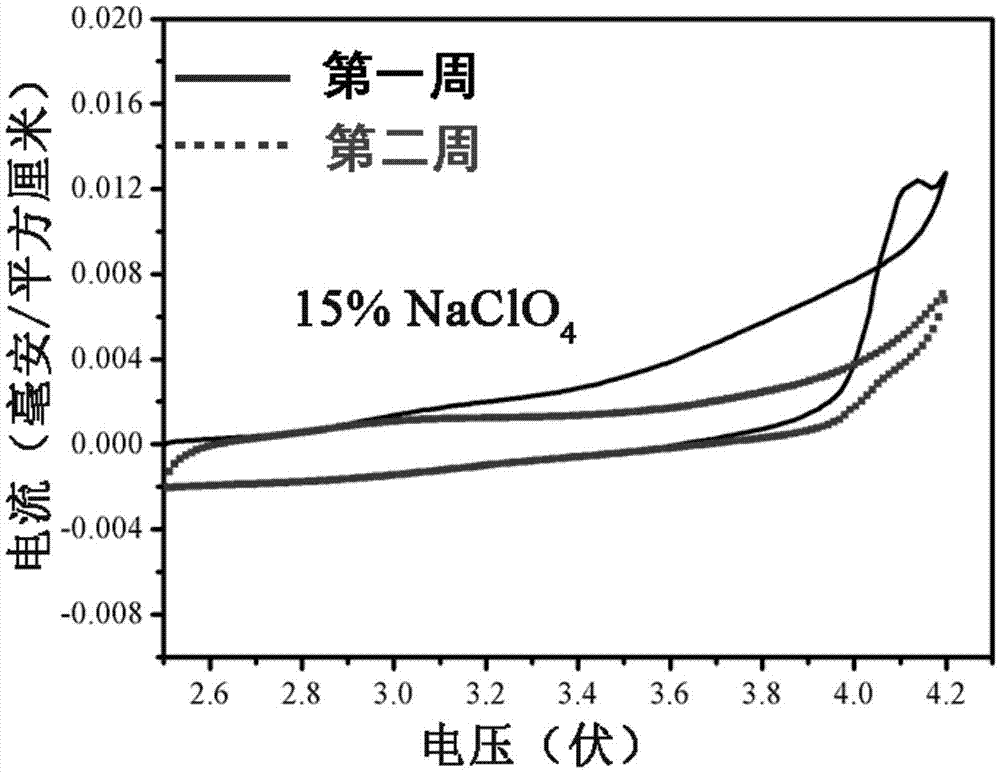
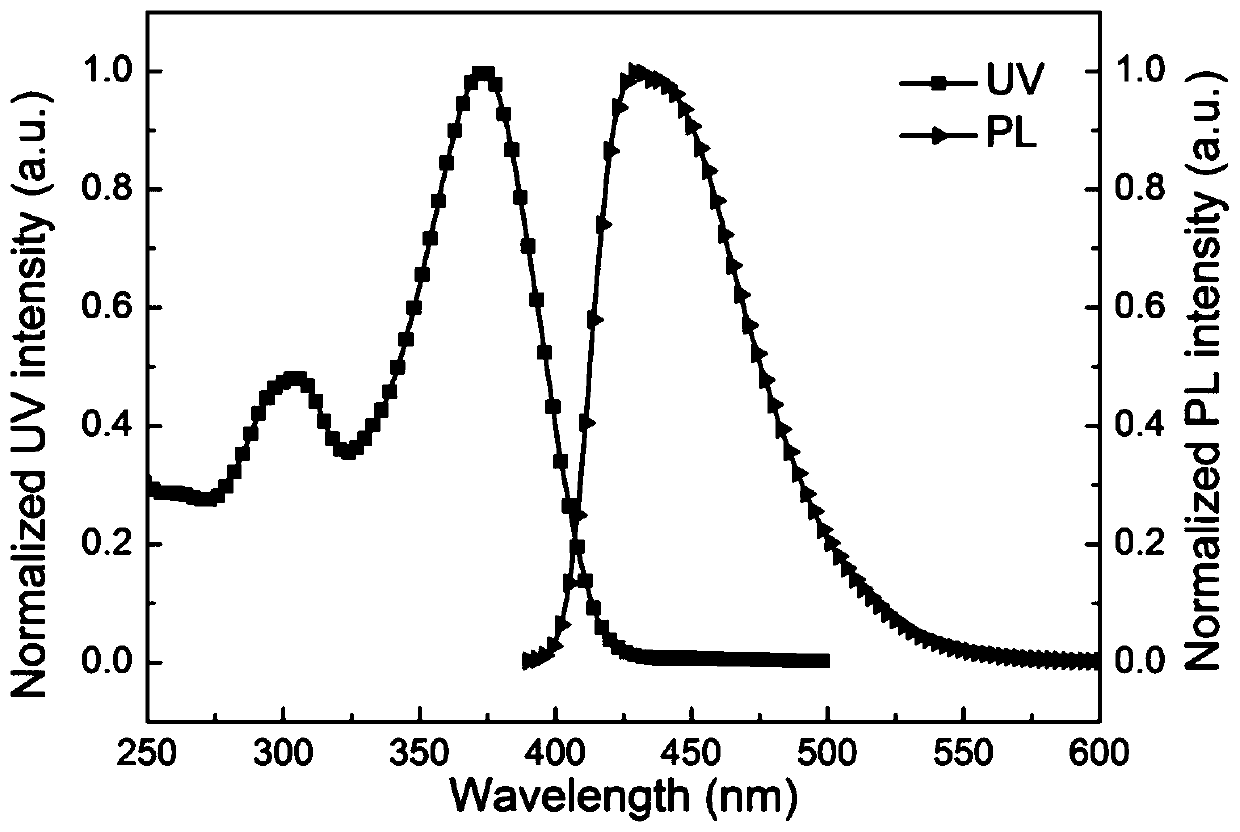
Patents
Literature
57 results about "Sodium hexafluorophosphate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
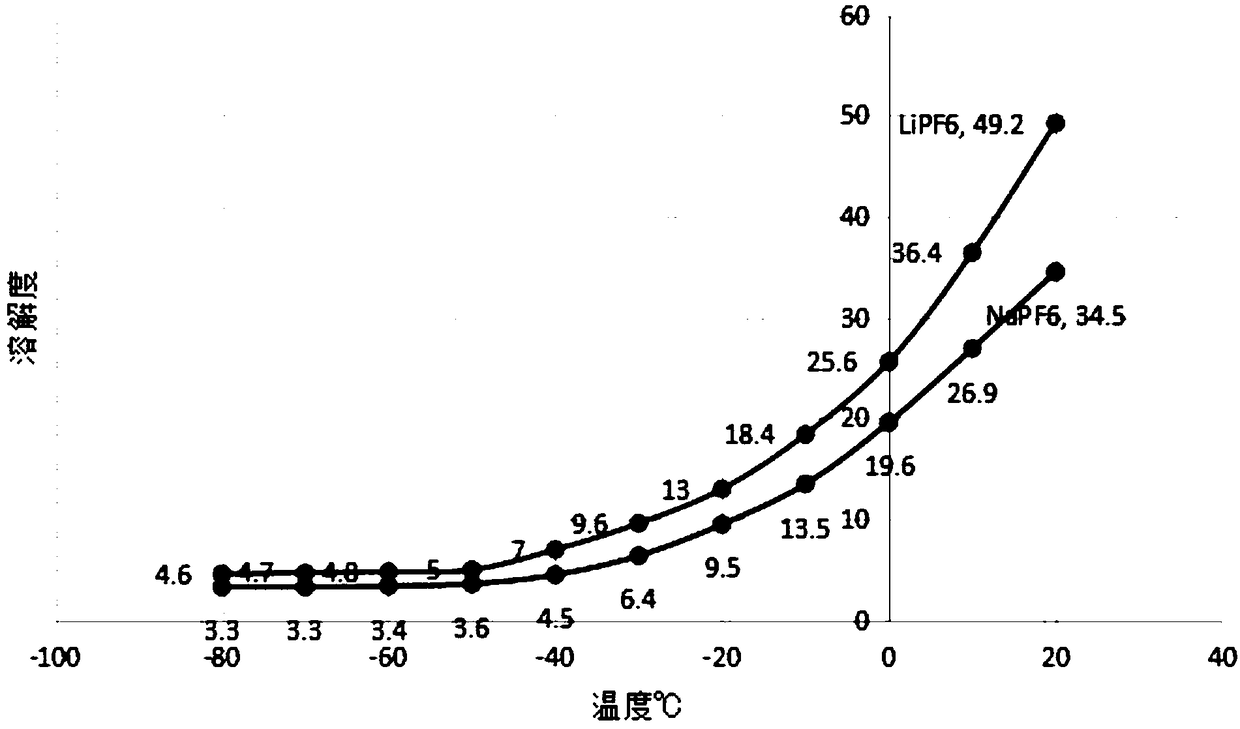
Sodium hexafluorophosphate is an inorganic compound with the chemical formula Na[PF₆]. It has recently been utilised as a component of non-aqueous electrolyte in rechargeable sodium-ion batteries.
Sodium ion battery negative electrode sheet and sodium ion battery
InactiveCN104966813AEnhanced insertion siteIncrease profitCell electrodesSecondary cellsElectrolytic agentSodium tetrafluoroborate
The present invention discloses a sodium ion battery negative electrode sheet, the negative electrode sheet is a porous graphite film structure, the diameter of the pores is from 2 to 30 microns, the distance between the centers of the circles of the pores is 5 to 50 microns, mass ratio of carbon atoms in the porous graphite film is greater than 99%, the negative electrode sheet may be used directly as the sodium ion battery negative electrode sheet, can avoid the use of a conductive agent, a binder and a metal collector, and has high capacity, corrosion resistance and good conductivity. The present invention also discloses a sodium ion battery using the negative electrode sheet, the sodium ion battery comprises a positive electrode sheet, a negative electrode sheet, a separator and an electrolyte, the sodium ion battery electrolyte solvent is one or more than one of diethanol dimethyl ether, dimethyl ether tetraethanol, and tetrahydrofuran, the electrolyte is one of sodium perchlorate, sodium hexafluorophosphate, sodium tetrafluoroborate and sodium trifluoromethanesulfonate, and the sodium ion battery is simple in production process and good in charging and discharging cycle stability, and has good prospects in the new energy field.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
Positive Electrode and Non-Aqueous Electrolyte Secondary Battery
ActiveUS20090053613A1Low costAlkaline accumulatorsNon-aqueous electrolyte accumulatorsElectrical batterySodium phosphates
A material (hereinafter referred to as “positive electrode material”) including sodium manganate powder as a positive electrode active material, carbon black powder as a conductive agent, and polytetrafluoroethylene as a binder is prepared. The positive electrode material is mixed in an N-methylpyrrolidone solution to produce slurry as a positive electrode mixture. A working electrode is produced by applying the slurry on a positive electrode collector. A negative electrode containing tin or germanium is produced. The non-aqueous electrolyte is produced by adding sodium hexafluorophosphate as an electrolyte salt in a non-aqueous solvent produced by mixing ethylenecarbonate and diethyl carbonate.
Owner:PANASONIC INTELLECTUAL PROPERTY MANAGEMENT CO LTD
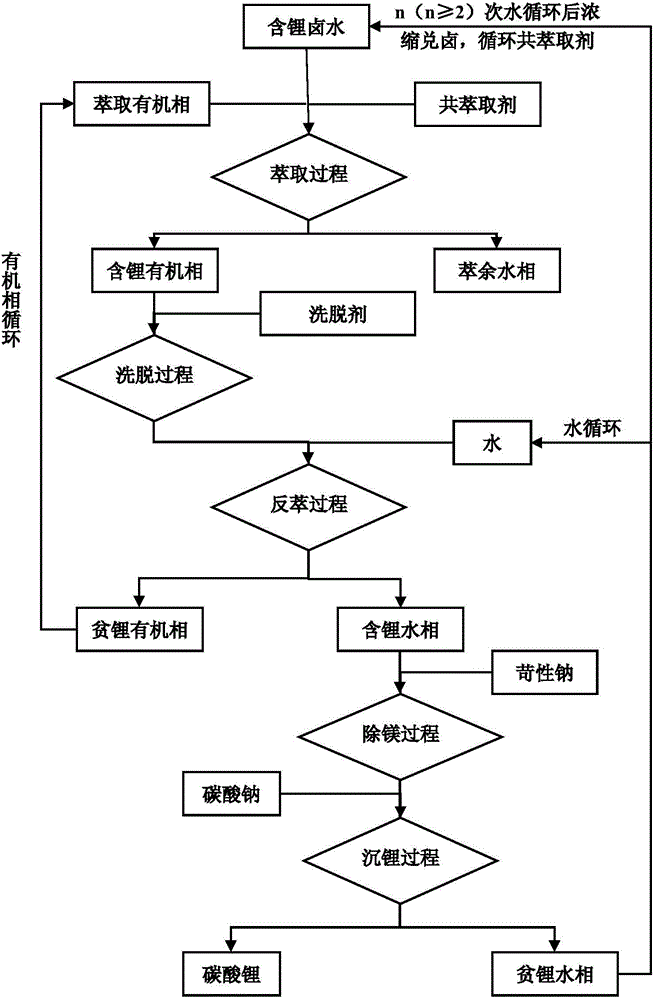
Novel co-extraction system for extraction of lithium from high magnesium-lithium ratio bittern and co-extraction method using the same
ActiveCN104404268AHigh extraction rateHigh stripping rateProcess efficiency improvementHigh magnesiumPotassium hexafluorophosphate
The invention discloses a novel co-extraction system for extraction of lithium from high magnesium-lithium ratio bittern. The novel co-extraction system comprises an extraction agent and a co-extraction agent. The co-extraction agent is a mixture of one or more of potassium hexafluorophosphate, sodium hexafluorophosphate, magnesium hexafluorophosphate, calcium hexafluorophosphate, potassium fluoborate, sodium fluoborate, magnesium fluoborate and calcium fluoborate. The novel co-extraction method for extraction of lithium from high magnesium-lithium ratio bittern comprises extraction, back extraction, deposition for magnesium removal and deposition for lithium acquisition, wherein aiming at bittern with a magnesium-lithium ratio greater than 100, an elution process is used between extraction and back extraction. The novel co-extraction system has simple processes and a high lithium extraction rate, effectively reduces a magnesium-lithium ratio of bittern, has obvious advantages than the traditional ferric trichloride extraction system, can be used for extraction of lithium from salt lake bittern and is especially suitable for solving the problem of high difficulty of extraction of lithium from salt lake bittern with a high magnesium-lithium ratio.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
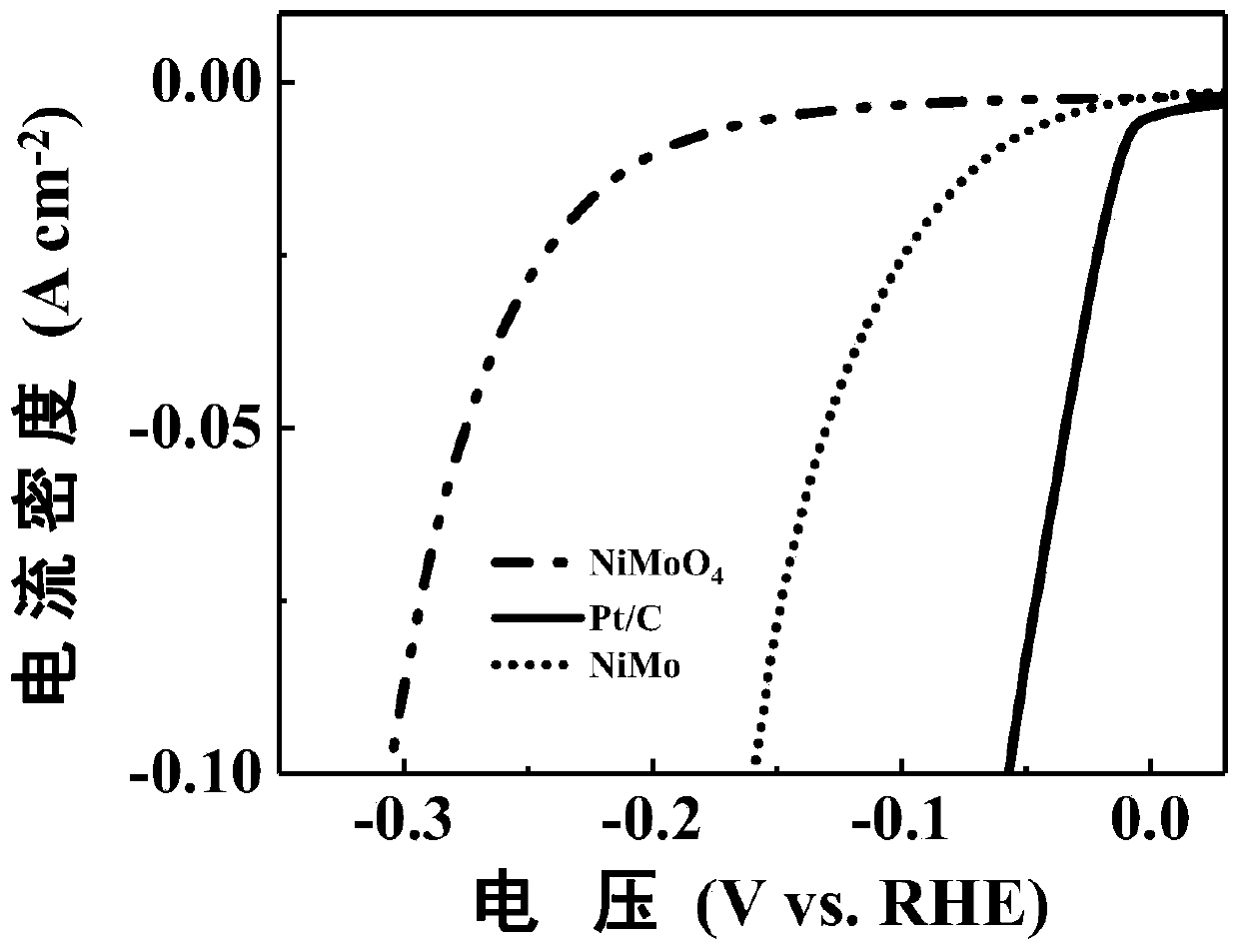
Preparation method of NiMo hydrogen evolution electrocatalyst
ActiveCN110227480APrecise Control of CompositionPerformance changes continuouslyMetal/metal-oxides/metal-hydroxide catalystsElectrodesNitrogen gasHeat treated
Belonging to the field of electrocatalytic hydrogen evolution, the invention discloses a preparation method of a NiMo hydrogen evolution electrocatalyst. The method includes: firstly preparing nickelnitrate hexahydrate and sodium molybdate dehydrate respectively into solutions; then transferring the two solutions into a reaction kettle, adding foamed nickel into the solutions, and carrying out hydrothermal reaction to obtain a nickel molybdate precursor material; then placing the prepared nickel molybdate precursor in a tubular furnace, and performing heat treatment in nitrogen atmosphere toobtain nickel molybdate; and finally, adopting nickel molybdate as the working electrode, taking a lithium sheet or sodium sheet as the counter electrode, using lithium hexafluorophosphate or sodium hexafluorophosphate as the electrolyte, conducting assembling to obtain a lithium battery or sodium battery, standing the battery for a set period of time, and then discharging the battery, thus obtaining the NiMo hydrogen evolution electrocatalyst. The method provided by the invention has no need for high temperature and strong reductant, has relatively mild process, and can precisely control thematerial composition.
Owner:HUAZHONG AGRI UNIV
Preparation method of sodium hexafluorophosphate
InactiveCN108946769AHigh purityIncrease productionSodium/potassium compoundsProcess equipmentSodium-ion battery
The invention discloses a preparation method of sodium hexafluorophosphate. The preparation method comprises the following steps: (1) introducing phosphorus pentafluoride gas into a reaction kettle containing lithium fluoride and hydrogen fluoride liquid for reaction, so as to obtain a sodium hexafluorophosphate solution; (2) carrying out crystallization under a stirring condition; and (3) drying.The preparation method has the advantages that the reaction conditions are mild, the yield is high, the process equipment is simple, and a high-purity sodium hexafluorophosphate target product can beprepared. Sodium hexafluorophosphate can be used as sodium salt for sodium-ion battery electrolyte.
Owner:MORITA NEW ENERGY MATERIALS ZHANGJIAGANG CO LTD
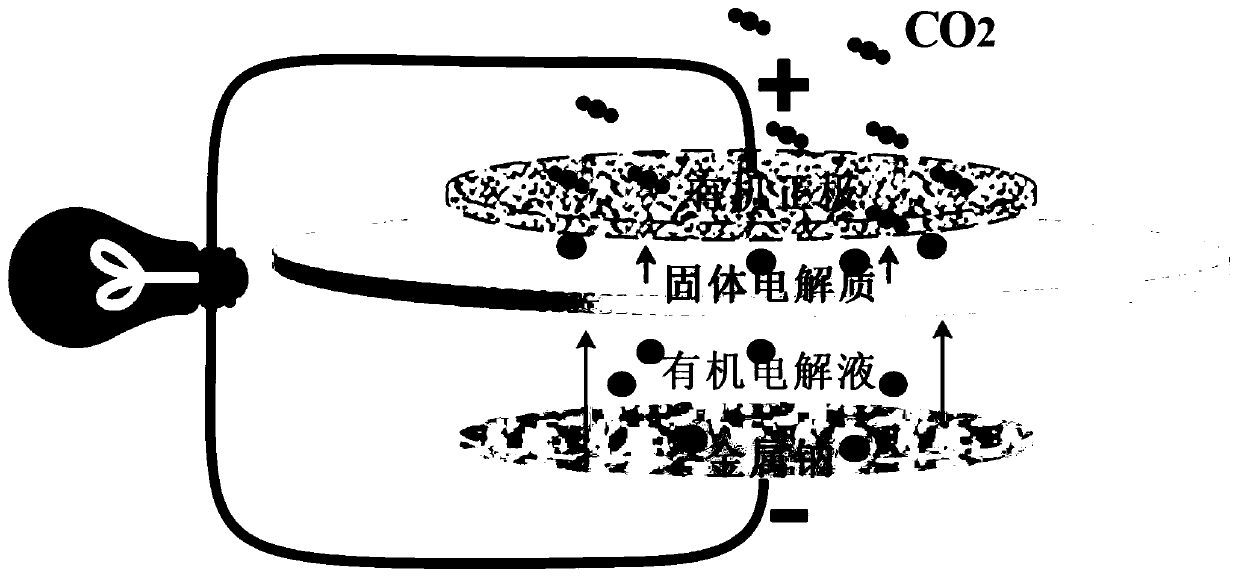
Organic anode used for sodium carbon dioxide battery and manufacturing method thereof, and sodium carbon dioxide battery
ActiveCN110534778AIncrease transfer rateImprove conductivityFuel and secondary cellsCell electrodesFiberCarbon fibers
The invention relates to an organic anode used for a sodium carbon dioxide battery and a manufacturing method thereof, and the sodium carbon dioxide battery. The organic anode comprises a catalyst, anorganic electrolyte for activating the catalyst and providing sodions, a conductive additive and a binder. The organic electrolyte comprises one of a sodium perchlorate electrolyte, a sodium trifluoromethanesulfonate electrolyte, a sodium bis(trifluoromethanesulfonyl)imide electrolyte and a sodium hexafluorophosphate electrolyte. The catalyst comprises at least one of graphite, activated carbon,Ketjen black, carbon fibers, carbon nano angles, carbon nanotubes, graphene, a modified carbon material and other porous active materials. The organic electrolyte activation catalyst can effectively accelerate a transmission rate of ions and electrons and conductivity is improved.
Owner:CENT SOUTH UNIV
Composite sodium-storage positive electrode for solid-state secondary sodium battery and preparation method for composite sodium-storage positive electrode
ActiveCN105489880AIncrease energy densityImprove securityCell electrodesSecondary cellsElectrical conductorElectrical battery
The invention relates to a composite sodium-storage positive electrode for a solid-state secondary sodium battery and a preparation method for the composite sodium-storage positive electrode. The composite sodium-storage positive electrode comprises an electrode active substance selected from any one kind of sodium manganate and sodium-titanium phosphate, and a sodium ion conductor selected from any one kind of sodium perchlorate and sodium hexafiuorophosphate. The preparation method is simple in preparation process and low in cost; and the prepared solid-state secondary sodium battery operating at the room temperature is high in energy density and high in safety.
Owner:SHANGHAI INST OF CERAMIC CHEM & TECH CHINESE ACAD OF SCI
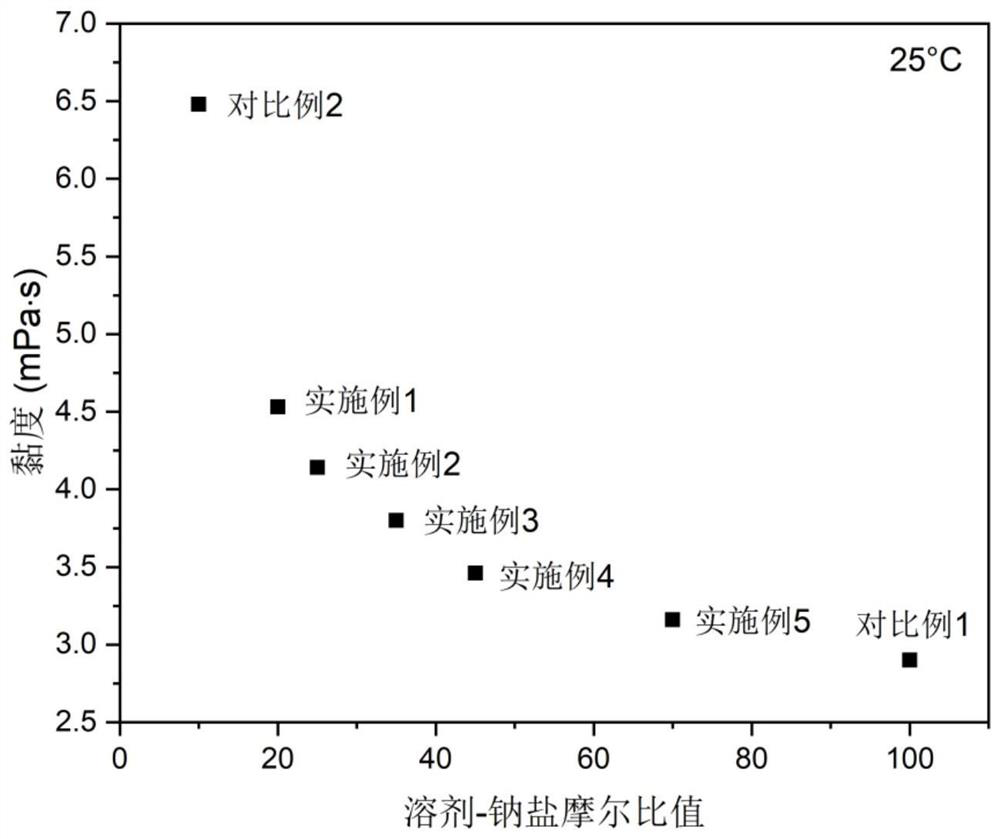
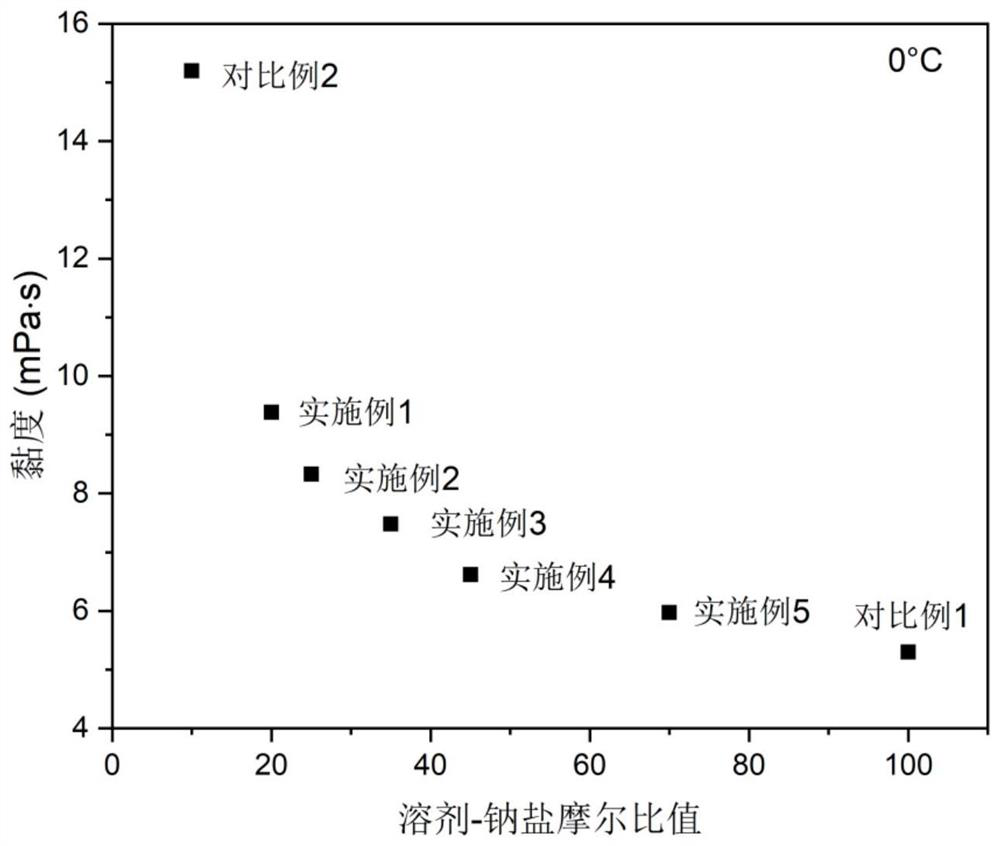
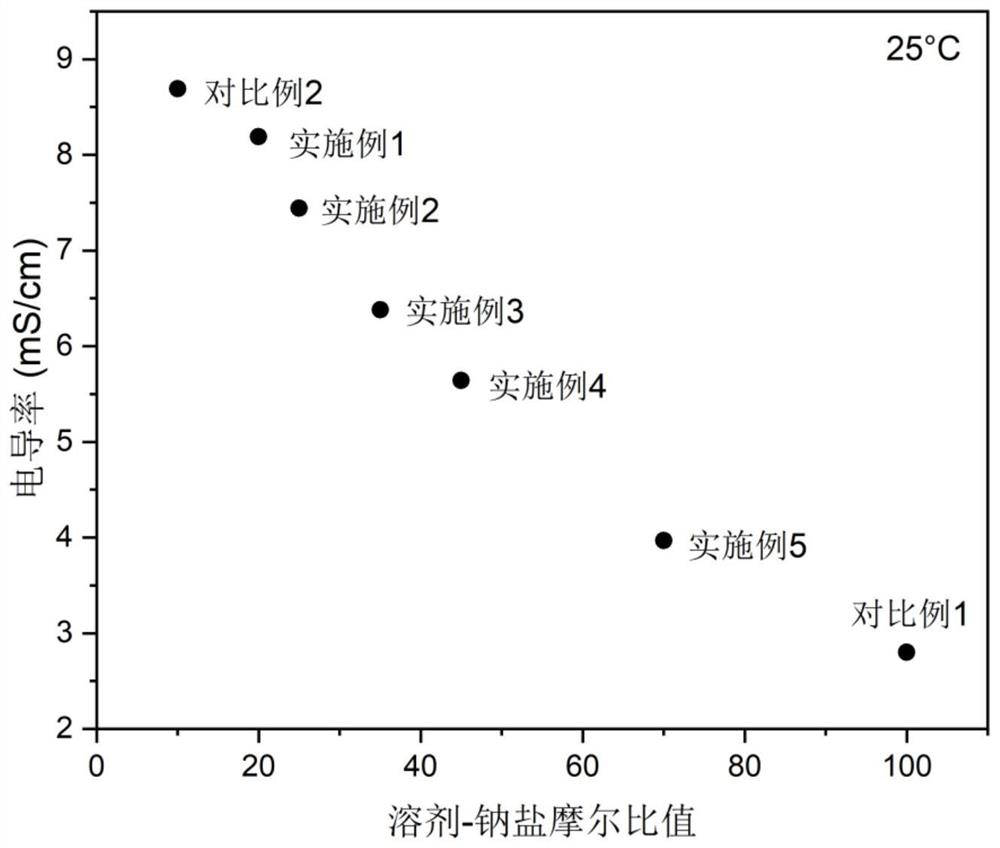
Electrolyte with high solvent-sodium salt ratio and sodium ion battery
PendingCN113299976AReduce viscosityImprove wettabilitySecondary cellsElectrolytic agentSodium tetrafluoroborate
The invention discloses an electrolyte with a high solvent-sodium salt ratio and a sodium ion battery. The electrolyte comprises sodium salt, a solvent and an additive, wherein the molar ratio of the solvent to the sodium salt is (20: 1)-(70: 1), and the addition amount of the additive accounts for 0.5%-5% of the mass of the electrolyte; the sodium salt comprises one or more of sodium perchlorate (NaClO4), sodium tetrafluoroborate (NaBF4), sodium hexafluorophosphate (NaPF6), sodium hexafluoroarsenate (NaAsF6), sodium trifluoroacetate (CF3COONa), sodium tetraphenylborate (NaB(C6H5)4), sodium trifluoromethanesulfonate (NaSO3CF3), sodium bis (fluorosulfonyl) imide (Na[(FSO2)2N]) or sodium bis (trifluoromethanesulfonyl) imide (Na[(CF3SO2)2N]).
Owner:INST OF PHYSICS - CHINESE ACAD OF SCI
Preparation method of sodium hexafluorophosphate
PendingCN114873577AReduce dependenceHigh puritySodium/potassium compoundsPhosphorus compoundsSodium phosphatesPhosphoric acid
The invention discloses a preparation method of sodium hexafluorophosphate, which comprises the following steps: step 1, dissolving phosphorus pentoxide in a certain proportion in a hydrogen fluoride solution to generate a hexafluorophosphoric acid solution; step 2, slowly adding a sodium source in a certain proportion into the hexafluorophosphoric acid solution prepared in the step 1; step 3, heating the solution after the reaction in the step 2, and removing water and unreacted hydrogen fluoride by nitrogen stripping; and 4, heating and concentrating the mother liquor obtained in the step 3, dissolving the obtained sodium hexafluorophosphate crude product into a poor solvent for recrystallization, and cooling and drying to obtain sodium hexafluorophosphate with the purity of 99.87% or above. According to the method, sodium hexafluorophosphate is synthesized, reactants are a hydrogen fluoride solution, phosphorus pentoxide and a high-purity sodium source, common impurities in industrial production do not exist, the purity is high, raw materials are easy to obtain, the reaction is simple, the reaction process is easy to control, few three wastes are generated, the obtained product can be applied to a sodium ion battery, and dependence on a lithium ion battery is relieved.
Owner:JIANGSU XINTAI MATERIALS TECH CO LTD
Low-temperature synthesis method of high-purity sodium hexafluorophosphate
PendingCN114772614AHigh purityMild conditionsSodium/potassium compoundsOrganic solventSodium phosphates
The invention discloses a low-temperature synthesis method of high-purity sodium hexafluorophosphate, which comprises the following steps: dissolving sodium fluoride in an organic solvent solution of Freon to obtain a solution; introducing phosphorus pentafluoride gas into the solution, carrying out low-temperature vacuum reaction for a set time, recovering to normal pressure, and evaporating Freon and the organic solvent to obtain sodium hexafluorophosphate; the reaction temperature of the low-temperature vacuum is-50 to 10 DEG C, and the reaction pressure is-0.2 to 0 MPa. Freon is adopted as a solvent, conditions are mild, the process is simple, and the cost can be effectively reduced; in addition, the Freon liquid can be evaporated by controlling the pressure, and the purity of the sodium hexafluorophosphate can be greatly improved. The reaction process and the subsequent solvent evaporation process are both carried out at low temperature, so that the decomposition of sodium hexafluorophosphate at high temperature can be effectively avoided, and the yield is improved.
Owner:SHANDONG UNIV
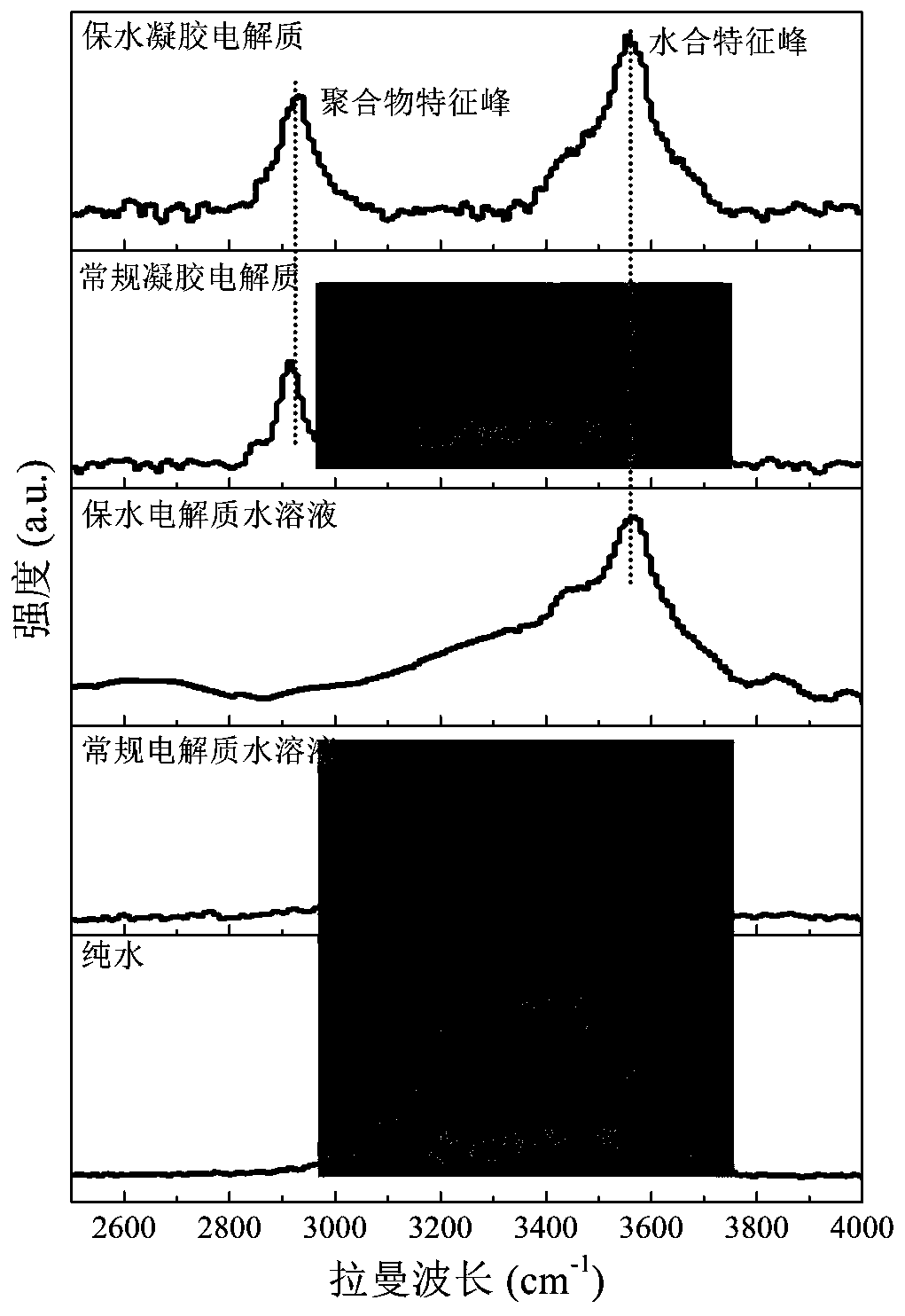
Water-retention gel electrolyte, preparation method thereof, and water-based supercapacitor and preparation method and application thereof
InactiveCN110246705AEasy to manufactureImprove water retentionHybrid capacitor electrolytesDouble layer capacitorsWater basedPotassium hexafluorophosphate
The invention provides a water-retention gel electrolyte , a preparation method thereof, and a water-based supercapacitor and a preparation method and application thereof, and relates to the field of gel electrolytes and supercapacitors. The water-retention gel electrolyte provided by the invention is prepared from an electrolyte aqueous solution and an oxygen-containing functional group polymer. The electrolyte in the electrolyte aqueous solution is one or more of lithium perchlorate, bis(trifluoromethane)sulfonimide lithium salt, lithium hexafluorophosphate, lithium nitrate, sodium chloride, sodium perchlorate, bis(trifluoromethane)sulfonimide sodium salt, sodium hexafluorophosphate, sodium nitrate, potassium chloride, potassium perchlorate, bis(trifluoromethane)sulfonimide potassium salt, potassium hexafluorophosphate and potassium nitrate. The oxygen-containing functional group polymer is one or more of polyvinylpyrrolidone, polyethylene glycol, polyvinyl alcohol, carboxymethyl cellulose and polyacrylamide. The water-based supercapacitor based on the water-retention gel electrolyte has ultra-long cycle life at the normal temperature, and can be normally charged and discharged at the high temperature of 120 DEG C and stably circulated.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for separating and detecting daclatasvir hydrochloride and optical isomers thereof
ActiveCN108732280AQuality improvementEfficient separationComponent separationPotassium hexafluorophosphateCellulose
The invention in particular relates to a method for separating and detecting daclatasvir hydrochloride and optical isomers thereof. The method for separating and determining daclatasvir hydrochlorideand optical isomers thereof (impurities) by using liquid chromatography is characterized by comprising the following steps: adopting a chiral chromatographic column taking cellulose tris(3,5-dimethylphenylcarbamate) as a filler, and taking a mixed solution which takes sodium hexafiuorophosphate, potassium hexafluorophosphate, formic acid, acetic acid, phosphoric acid or aqueous phosphate solutionas an aqueous phase and takes acetonitrile or methanol as an organic phase as a mobile phase. According to the separating and detecting method disclosed by the invention, the daclatasvir hydrochlorideand optical isomers thereof (impurities) can be effectively separated, the degree of separation reaches 3.0 or higher, and complete baseline separation is realized, so that the quality of the daclatasvir hydrochloride can be accurately and effectively controlled. With the adoption of the separating method disclosed by the invention, the time of separating and detecting the daclatasvir hydrochloride and optical isomers thereof is within 30-80 minutes. The method disclosed by the invention has the advantages of being simple, rapid, accurate and the like.
Owner:SUNSHINE LAKE PHARM CO LTD
Sodium ion secondary battery and non-aqueous electrolyte therefor
InactiveCN106960978AAvoid corrosionCorrosion does not occurSecondary cellsOrganic electrolytesImidePhysical chemistry
The invention discloses a non-aqueous electrolyte for a sodium ion secondary battery and with a function of restraining aluminum foil corrosion of a positive electrode current collector. The non-aqueous electrolyte comprises sodium bis(fluorosulfonyl)imide, a functional additive sodium perchlorate and / or sodium hexafiuorophosphate. The invention also discloses the sodium ion secondary battery comprising the non-aqueous electrolyte, and a preparation method and an application of the sodium ion secondary battery.
Owner:INST OF PHYSICS - CHINESE ACAD OF SCI
Organelle-targeted aggregation-induced emission material and preparation method thereof
ActiveCN110668997AThe synthesis process is simpleSimple purification processOrganic chemistryFluorescence/phosphorescencePtru catalystSodium phosphates
The invention discloses an organelle-targeted aggregation-induced emission material and a preparation method thereof. The method comprises the following steps: 1) taking a triphenylamine borate and anaryl bromide compound as raw materials, fully and completely dissolving in a toluene solution, adding a potassium carbonate solution and ethanol solvent, taking tetrakis (triphenylphosphine) palladium as a reaction catalyst, carrying out nitrogen reaction, adding extraction, washing, removing of solvent, and carrying out column chromatography purification to obtain a target material; wherein whenthe aryl bromide compound in the step 1 is 2,5-dibromopyridine and 2, 5-dibromopyrazine, a product obtained in the step 1 and methyl iodide are taken as raw materials and are dissolved in toluene,heating reaction is carried out, toluene solvent is removed, then sodium hexafluorophosphate and acetonitrile are added to be fully dissolved, stirring is carried out for 12-24 hours at the room temperature, and a target fluorescent product can also be obtained after water washing, solvent removal under reduced pressure and recrystallization. The preparation and purification cost is low; the producthas excellent fluorescence emission performance, and can realize specific targeting imaging performance research of a plurality of organelles in cells.
Owner:XI AN JIAOTONG UNIV
Non-aqueous electrolyte for inhibiting sodium-ion battery swelling as well as preparation method and application thereof
InactiveCN108288730ASuppress flatulenceImprove Coulombic efficiencyFinal product manufactureSecondary cells servicing/maintenanceSodium tetrafluoroborateSodium-ion battery
The invention discloses non-aqueous electrolyte for inhibiting sodium-ion battery swelling as well as a preparation method and application thereof. The non-aqueous electrolyte comprises a sodium salt,an organic solvent and a first functional additive, wherein the molecular formula of the first functional additive is R1R2R3C3H3O3S; the content of the first functional additive in the non-aqueous electrolyte is 0.001-30wt%; the sodium salt refers to one or more of sodium hexafluorophosphate, sodium tetrafluoroborate, sodium hexafluoroarsenate, sodium borate dioxalate, sodium ditrifluoromethyl sulfonamide, sodium trifluoromethanesulfonate, sodium difluorosulfonimide or sodium perchlorate; the organic solvent refers to one or more of cyclic carbonate, chain linear carbonate, carboxylic ester or cyclic lactone.
Owner:INST OF PHYSICS - CHINESE ACAD OF SCI
Preparation method of sodium salt for sodium battery
ActiveCN114751431AIncrease profitIncrease productionCell electrodesSodium/potassium compoundsPolyvinyl alcoholSodium phosphates
The invention discloses a preparation method of a sodium salt for a sodium battery, which comprises the following steps: uniformly mixing polyvinyl alcohol and sodium fluoride, adding deionized water, drying, and calcining to obtain porous sodium fluoride; the method comprises the following steps: introducing phosphorus pentafluoride gas into a mixture of porous sodium fluoride and hydrogen fluoride liquid, and reacting to prepare a sodium hexafluorophosphate solution; and crystallizing, filtering and drying the sodium hexafluorophosphate solution to obtain the sodium hexafluorophosphate. Porous sodium fluoride is adopted as a sodium source to prepare sodium hexafluorophosphate, the utilization rate of a precursor can be increased, and the yield is effectively increased; in addition, the yield of the product can be further improved through stirring and crystallization.
Owner:SHANDONG UNIV
High-strength welding clamp surface treatment method
InactiveCN108326749AHigh strengthImprove wear resistanceCleaning using liquidsSodium phosphatesSodium polysulfide
The invention discloses a high-strength welding clamp surface treatment method. The high-strength welding clamp surface treatment method comprises the following operation steps that firstly, a hydrochloric acid solution is evenly scattered to the surface of a welding clamp, 10 minutes later, the surface is washed through clean water, and pre-shot blasting liquid is used for being evenly sprayed tothe surface of the welding clamp, wherein the pre-shot blasting liquid is composed of, by weight, 9-13 parts of sodium phosphate, 5-7 parts of ammonium hydroxide, 2-4 parts of benzyl trimethyl ammonium iodide, 1-3 parts of sodium hexafluorophosphate, 4-6 parts of sodium polysulfide and 190-200 parts of water; and secondly, a peening machine is used for spraying ceramic pills to the surface of thewelding clamp, wherein the average diameter of the ceramic pills is 500 microns, the spraying angle is 90 degrees, and the distance between a nozzle and a sample is 180 mm. By means of the high-strength welding clamp surface treatment method, by treating the surface of the welding clamp, the strength of the welding clamp can be effectively improved, the abrasion resisting performance of the welding clamp is greatly improved, and the service life of the welding clamp is effectively prolonged.
Owner:安徽省明升机械科技有限公司
Electrolytic preparation method for composite phosphating film
ActiveCN107630243AShort processing timeHigh film forming efficiencyPhosphatisationRare-earth elementElectrolysis
The invention discloses an electrolytic preparation method for a composite phosphating film. A composite phosphating film treating fluid mainly consists of phosphoric acid, nitric acid, zinc oxide, nickel sulfate, sodium phytate, sodium hexafluorophosphate, succinic acid, ammonium phosphomolybdate, hydroxylamine sulphate, cerium nitrate, hydrogen peroxide and water, and can be used for cold machining and machine manufacturing. According to the preparation for the composite phosphating film disclosed by the invention, an inert material is taken as a working electrode, and a special current applying method is adopted to treat to form an organic phosphating-inorganic phosphating composite protective layer which is excellent in performance and is doped with rare-earth elements on the surface of a workpiece substrate. During operation and use, the electrolytic preparation method has the characteristics of a medium-low temperature, short treatment time and high film-forming efficiency; and atreated workpiece has excellent stretching ductility and excellent extruding property, and can be popularized to various precise forming pre-treatment processes.
Owner:ZHEJIANG WUYUAN TECH CO LTD
Preparation method of high-performance fishing net
InactiveCN107503119AGood mechanical propertiesImprove qualityStain/soil resistant fibresFishing netEnvironmentally friendly
The invention discloses a preparation method of a high-performance fishing net. The preparation method comprises operating steps as follows: (1), after being soaked with hot water for 3-5 h, the fishing net formed through weaving is immediately placed in a soaking solution at the temperature of 0 DEG C for 20-30 min, and the soaking solution is prepared from components in parts by weight as follows: 15-19 parts of sodium hexafluorophosphate, 7-10 parts of 1-chlorooctane, 6-8 parts of ammonium pentaborate and 220-250 parts of water; (2), after being taken out, the fishing net is soaked in a modifier for 6-8 h and then is dried, and a finished product is obtained. The preparation method of the high-performance fishing net is simple to operate, low in cost, safe and environmentally friendly, the raw materials are easily available, large-scale industrial production can be realized, the prepared high-performance fishing net is excellent in mechanical performance and anti-oxidative, particularly, the treated fishing net is free of odorous pungent smell, and the quality of the fishing net is greatly improved.
Owner:CHAOHU JUNYE FISHING TACKLE CO LTD
Positive electrode and non-aqueous electrolyte secondary battery
ActiveUS8815449B2Low costAlkaline accumulatorsNon-aqueous electrolyte accumulatorsSlurryMaterials science
Owner:PANASONIC INTELLECTUAL PROPERTY MANAGEMENT CO LTD
Method for utilizing plastic bottles to prepare non-woven fabric
ActiveCN107287764AReduce manufacturing costGood mechanical propertiesNon-woven fabricsArtifical filament manufactureWarm waterEngineering
The invention discloses a method for utilizing plastic bottles to prepare non-woven fabric. The method includes the steps of (1) after the plastic bottles are cut into shreds, conducting treatment for removing taints and oil, putting the plastic bottle shreds into warm water, adding sodium hexafluorophosphate into the water, and taking out the plastic bottle shreds to conduct drying treatment after 25-35 min; (2) after the dried plastic bottle shreds are blended with addictives, conducting prilling, and then conducting melt-blow spinning to obtain finished products, wherein the weight ratio of the plastic bottle shreds to the addictives is 50-55 to 1. The method for utilizing the plastic bottles to prepare the non-woven fabric has the advantages of being simple in process procedures, capable of reducing production costs of the non-woven fabric effectively, and providing a new approach to development and utilization of waste plastic, and the non-woven fabric prepared according to the method is excellent in mechanical properties and long in service life.
Owner:MAANSHAN CITY XIN CHENG NANO NEW MATERIAL TECH CO LTD
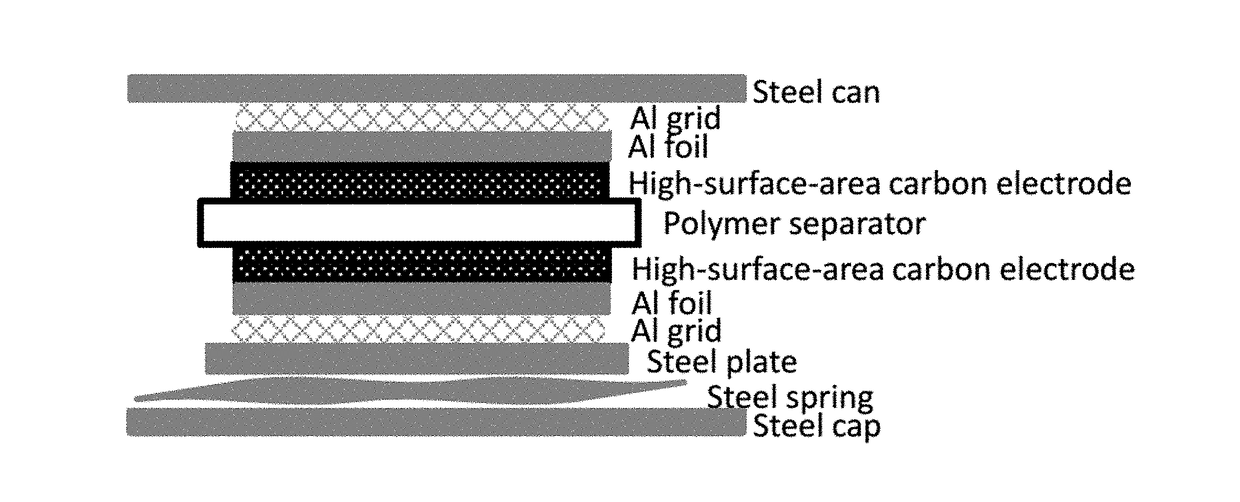
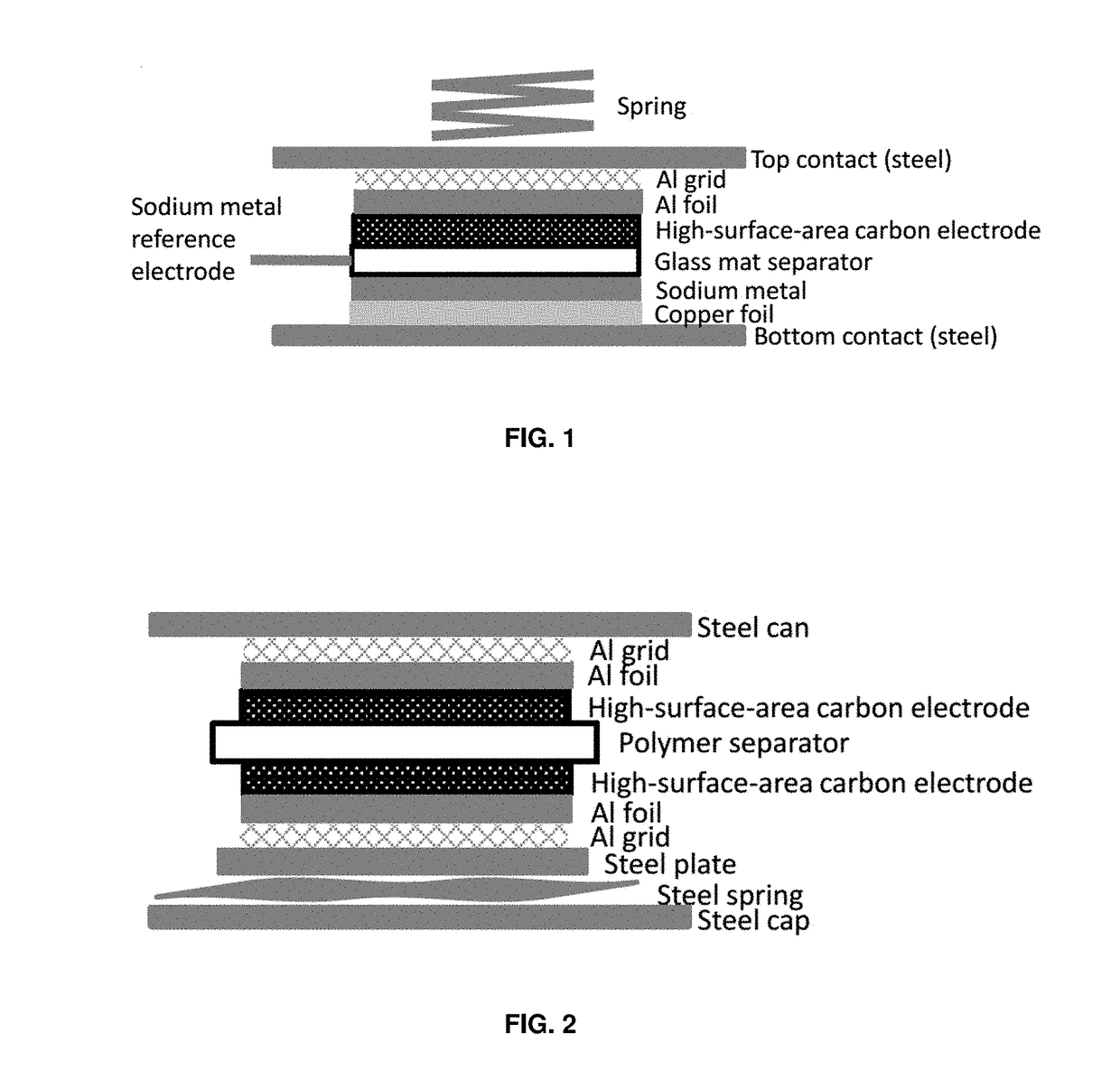
High voltage window electrolyte for supercapacitors
ActiveUS20180130611A1Hybrid capacitor electrolytesHybrid capacitor electrodesHigh pressureHigh voltage
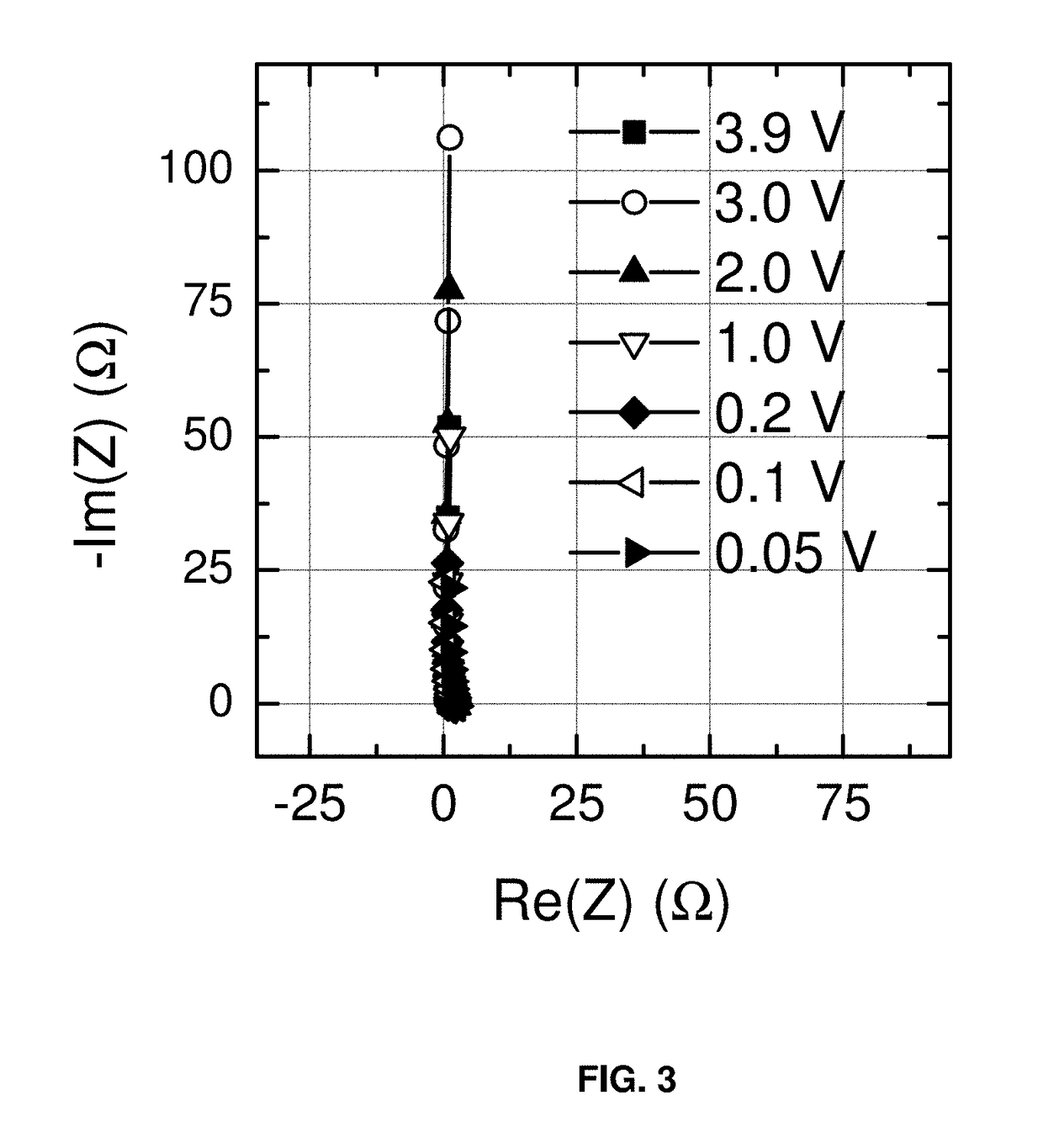
A supercapacitor according to the present invention includes a negative carbon-comprising electrode which does not intercalate sodium, and a positive carbon-comprising electrode. An electrolyte composition comprises sodium hexafluorophosphate and a non-aqueous solvent comprising at least one selected from the group consisting of ethylene glycol dimethyl ether, diethylene glycol dimethyl ether, triethylene glycol dimethyl ether, and tetraethylene glycol dimethyl ether. The supercapacitor has an electrochemical voltage window of from +0.0 V to 3.5 V (full cell voltage). The electrolyte has an electrochemical voltage window of from +0.05 V to 3.9 V vs. Na / Na+. A method of making and a method of operating a supercapacitor is also disclosed.
Owner:UT BATTELLE LLC
Negative electrode and non-aqueous electrolyte secondary battery using the same
InactiveUS20100015532A1Low costNon-aqueous electrolyte accumulatorsElectrode carriers/collectorsSolventCopper
A rolled foil of surface roughened copper as thick as 26 μm for example having a surface formed into an irregular shape with copper precipitated thereon by an electrolytic method is prepared as a negative electrode collector. Tin (Sn) or germanium (Ge) is deposited on the rolled foil described above, so that a negative electrode active material layer is formed. Note that the deposited tin or germanium is amorphous. The arithmetic mean roughness Ra in the surface-roughened rolled foil described above is preferably not less than 0.1 μm nor more than 10 μm. A non-aqueous electrolyte is produced by adding sodium hexafluorophosphate as an electrolyte salt in a concentration of 1 mol / l to a non-aqueous solvent produced by mixing ethylene carbonate and diethyl carbonate in the ratio of 50:50 by volume.
Owner:PANASONIC INTELLECTUAL PROPERTY MANAGEMENT CO LTD
Additive for sodium-ion battery electrolyte, electrolyte and sodium-ion battery
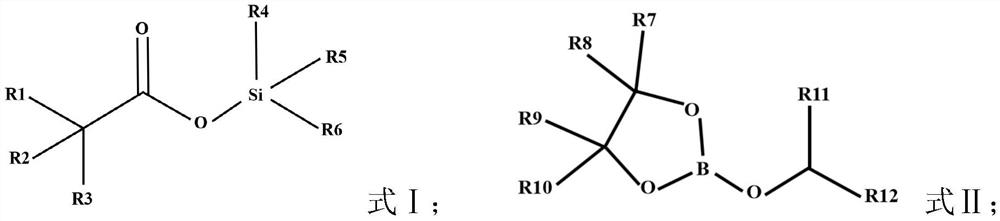
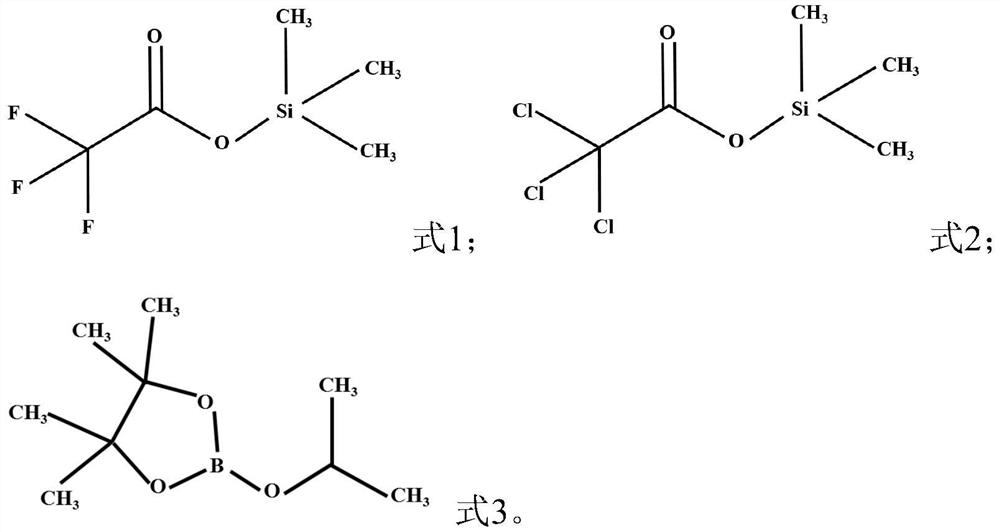
PendingCN113921907AImprove electrochemical performanceImprove cycle performanceSecondary cellsOrganic electrolytesElectrolytic agentHydrofluoric acid
The invention provides an additive for a sodium-ion battery electrolyte, the electrolyte and a sodium-ion battery, the additive comprises a first additive and a second additive, the first additive is a compound as shown in a formula I, and the second additive is a compound as shown in a formula II. Compared with the prior art, the sodium-ion battery electrolyte comprises at least two additives, so that the cycle performance and the rate capability of the battery can be obviously improved. Specifically, the first additive can spontaneously react with trace water and hydrofluoric acid in the electrolyte, so that the hydrolysis of sodium hexafluorophosphate is inhibited, the corrosion of hydrofluoric acid, phosphorus pentafluoride and other products on the positive electrode material is prevented, the stability of the material and the electrolyte is improved, and the electrochemical performance of the sodium-ion battery is improved; the second additive can form a stable interfacial film on the surfaces of the positive electrode and the negative electrode, and an organic sodium-rich interfacial film formed on the positive electrode can effectively inhibit transition metal in the positive electrode from being dissolved into electrolyte, so that the loss of active substances is reduced, and the resistance is improved.
Owner:SO-FUN TECH CORP LTD
Bipolar sodium ion battery and preparation method thereof
ActiveCN114094170AInhibit sheddingIncrease profitFinal product manufactureElectrode carriers/collectorsElectrical batterySodium phosphates
The invention relates to a bipolar sodium ion battery and a preparation method thereof, the bipolar sodium ion battery comprises a plurality of bipolar pole pieces, a diaphragm and an electrolyte, the plurality of bipolar pole pieces are sequentially stacked, each bipolar pole piece comprises an aluminum foil, an insulating strip and an insulating layer, the aluminum foil is provided with a first surface and a second surface which are oppositely arranged, each of the first surface and the second surface comprises a positive electrode region, a bending region and a negative electrode region which are sequentially arranged, the positive electrode region is coated with a positive electrode active layer, and the negative electrode region is coated with hard carbon; the positive electrode active layer comprises a transition metal oxide, a Prussian blue material, a polyanion compound or an amorphous material; the aluminum foils can be folded along the bending area, so that the positive electrode region and the negative electrode region are arranged oppositely; the insulating strip is arranged in the bending region; the insulating layer is clamped between the positive active layer and the hard carbon; a diaphragm is arranged between every two adjacent bipolar pole pieces; and the electrolyte is used for conducting electricity for the positive electrode active layer and the hard carbon and comprises sodium hexafluorophosphate. The bipolar sodium ion battery is high in energy density and long in service life.
Owner:深圳市睿赛新能源科技有限公司 +1
Method for electrolytic wax removal from automobile covering part
The invention discloses a method for electrolytic wax removal from an automobile covering part. The method comprises the following operation steps: 1, an electrolytic wax removal solution is prepared, 4-trimethylsilyl styrene, sodium methallyl sulfonate, diethylene glycol chloroformate, a nonionic surfactant, a diffusion agent, tetrabutyl ammonium fluoride, sodium hexafluorophosphate and water are uniformly mixed, and then are continuously stirred for 30-40 minutes by adopting the rotate speed of 400-500 r / min; and 2, electrolysis is carried out, a workpiece is subjected to cathode electrolysis for 2-4 minutes, and is subjected to anode electrolysis for 1-2 minutes, then the workpiece is washed by hot water at the temperature of 50-60 DEG C, and the electrolytic wax removal is completed. The method for the electrolytic wax removal from the automobile covering part has the advantages that the electrolytic wax removal solution is green and environment-friendly, the wax removal effect is remarkable so that the wax on the workpiece surface can fall off quickly, and the service life of the wax removal solution can be effectively prolonged.
Owner:阜南县申通机械制造有限公司
A kind of preparation method of lithium ion battery
ActiveCN109786836BImprove cycle performanceLower internal resistanceFinal product manufactureSecondary cells charging/dischargingElectrolytic agentSodium phosphates
The invention provides a preparation method of a lithium-ion battery. The electrolyte of the lithium-ion battery includes ethylene sulfate and sodium hexafluorophosphate as additives, wherein ethylene sulfate accounts for 0.5-3% of the total volume of the electrolyte. %, the concentration of sodium hexafluorophosphate in the electrolyte is 0.001-0.02mol / L, wherein the formation method includes the first step of formation under the atmosphere of high concentration carbon dioxide, and under the atmosphere of low concentration or zero concentration carbon dioxide In the second step of chemical formation, a stable SEI film is formed by the method of the present invention, which avoids an increase in internal resistance and reduces the volume expansion rate of the battery after cycling, especially in a high-temperature environment.
Owner:金明信(北京)科技有限公司
A method for producing calcium fluoride from waste LCD panel glass etchant
InactiveCN104071820BHigh purityAccelerated settlementCalcium/strontium/barium fluoridesHexafluoropropyleneSodium aluminate
The invention relates to a method for producing calcium fluoride from waste LCD panel glass etching solution, which comprises the following steps in turn: take a certain amount of waste LCD panel glass etching solution, add sodium fluoride solid, and fully stir to make hexafluoride in the waste liquid Complete reaction of silicic acid and sodium fluoride to generate sodium hexafluorosilicate and hydrofluoric acid, complete reaction of hexafluoroaluminate and sodium fluoride to generate sodium hexafluoroaluminate and hydrofluoric acid, and then add ammonia water to react to generate silicon dioxide , sodium fluoride and ammonium bifluoride, so that sodium hexafluoroaluminate and the remaining sodium hexafluorosilicate settle rapidly, then filter the precipitate, take the supernatant and add it to the calcium hydroxide suspension until pH = 5 to stop, and make the hydrogen Calcium oxide completely reacts with hydrofluoric acid to generate calcium fluoride, then add calcium hydroxide suspension to make sodium fluoride and ammonium bifluoride react with calcium hydroxide to generate calcium fluoride, then add polyacrylamide solution, precipitate, filter and washing, and finally drying to obtain calcium fluoride solid. The purity of calcium fluoride can reach more than 95%.
Owner:YANGZHOU BRIGHTMAN INT
Anti-freezing agent for concrete and preparation method of anti-freezing agent
The invention discloses an anti-freezing agent for concrete and a preparation method of the anti-freezing agent. The anti-freezing agent for concrete is prepared from raw materials in parts by weightas follows: 12-18 parts of methylpentanolone, 8-12 parts of succinimide, 5.4-6.6 parts of mushroom alcohol, 4.6-6.2 parts of sodium hexafluorophosphate, 3.8-5.4 parts of tri-isopropanolamine, 2.4-4 parts of expanded perlite and 1.5-3.5 parts of N-vinyl pyrrolidone. The anti-freezing agent is prepared from methylpentanolone, succinimide, mushroom alcohol, sodium hexafluorophosphate, tri-isopropanolamine, expanded perlite and N-vinyl pyrrolidone with the preparation method, can normally work at the temperature of subzero 30 DEG C, notably improves the anti-freezing performance when used in concrete, can improve the compressive strength and water reduction performance of the concrete and has good compatibility with the concrete. The preparation method adopts a simple preparation process and is convenient to operate, prone to industrial production and worth popularization.
Owner:HENAN HUANQIU AVIATION EQUIP TECH CO LTD
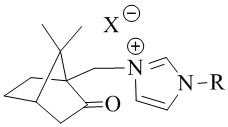
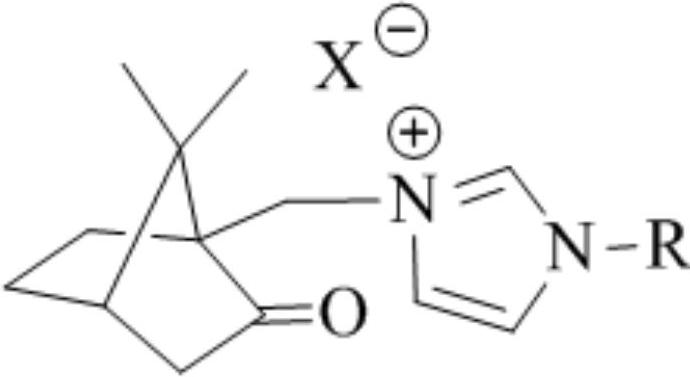
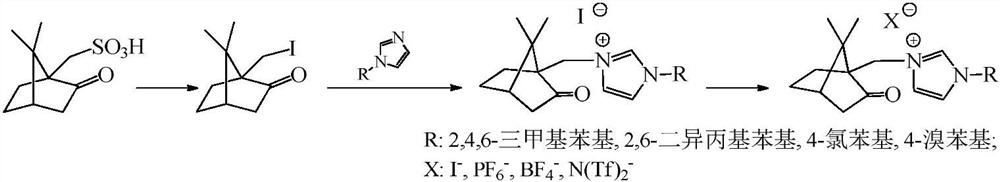
A class of camphor-based imidazole type ionic liquid and its preparation method and application
ActiveCN110272344BHigh catalytic efficiencyMild reaction conditionsCarboxylic acid nitrile preparationOrganic compound preparationSodium tetrafluoroborateSodium phosphates
The invention discloses a class of camphor-based imidazole type ionic liquid, its preparation method and application. The present invention uses camphorsulfonic acid, a derivative of camphor, which is a natural renewable resource, as a raw material to prepare 10-iodated camphor; 10-iodated camphor reacts with aryl imidazole in a quaternization reaction to obtain camphor-based imidazole iodized salt; camphor Ion-exchange imidazole iodide salt with sodium hexafluorophosphate, sodium tetrafluoroborate, bis(trifluoromethanesulfonimide) lithium, etc. to obtain camphor-imidazole hexafluorophosphate and camphor-imidazole tetrafluoroborate , Camphor-based imidazole bis (trifluoromethanesulfonimide) salt plasma liquid. Camphor-based imidazole-based ionic liquids exhibit good catalytic activity for the oxidative esterification of aldehyde-alcohols, and have the advantages of short reaction time, good reaction selectivity, and high product yield, and have good application prospects.
Owner:NANJING FORESTRY UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com