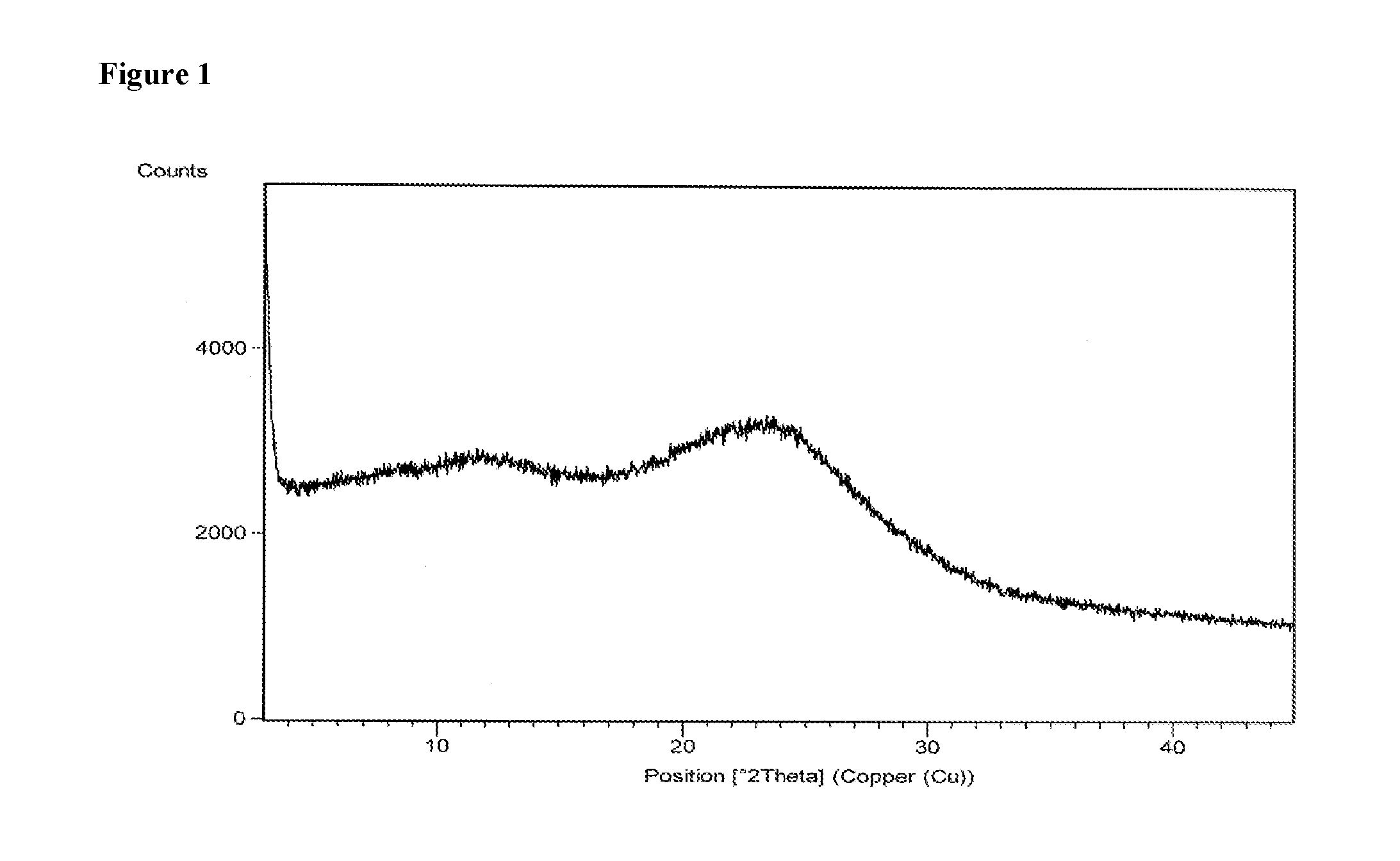
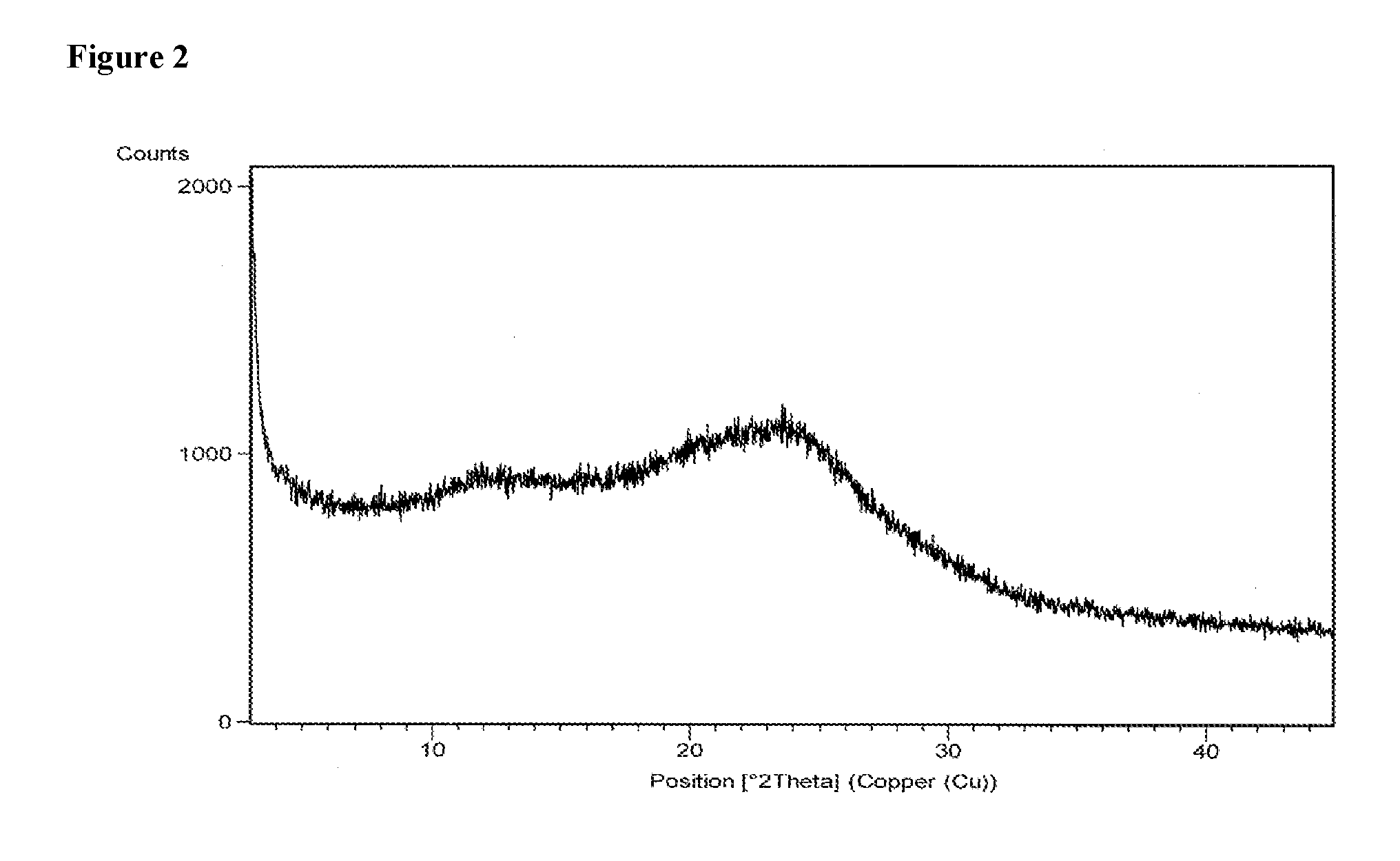
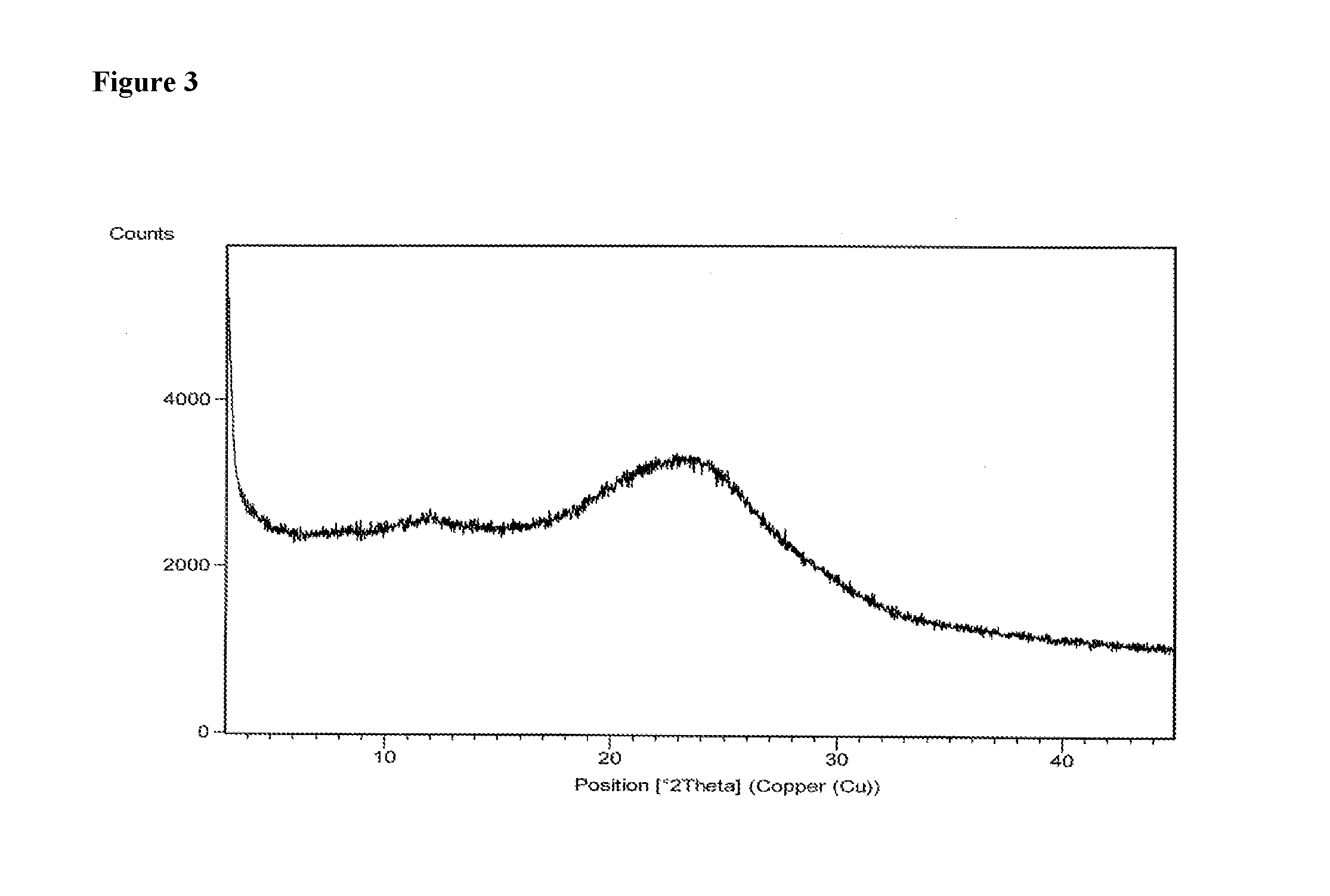
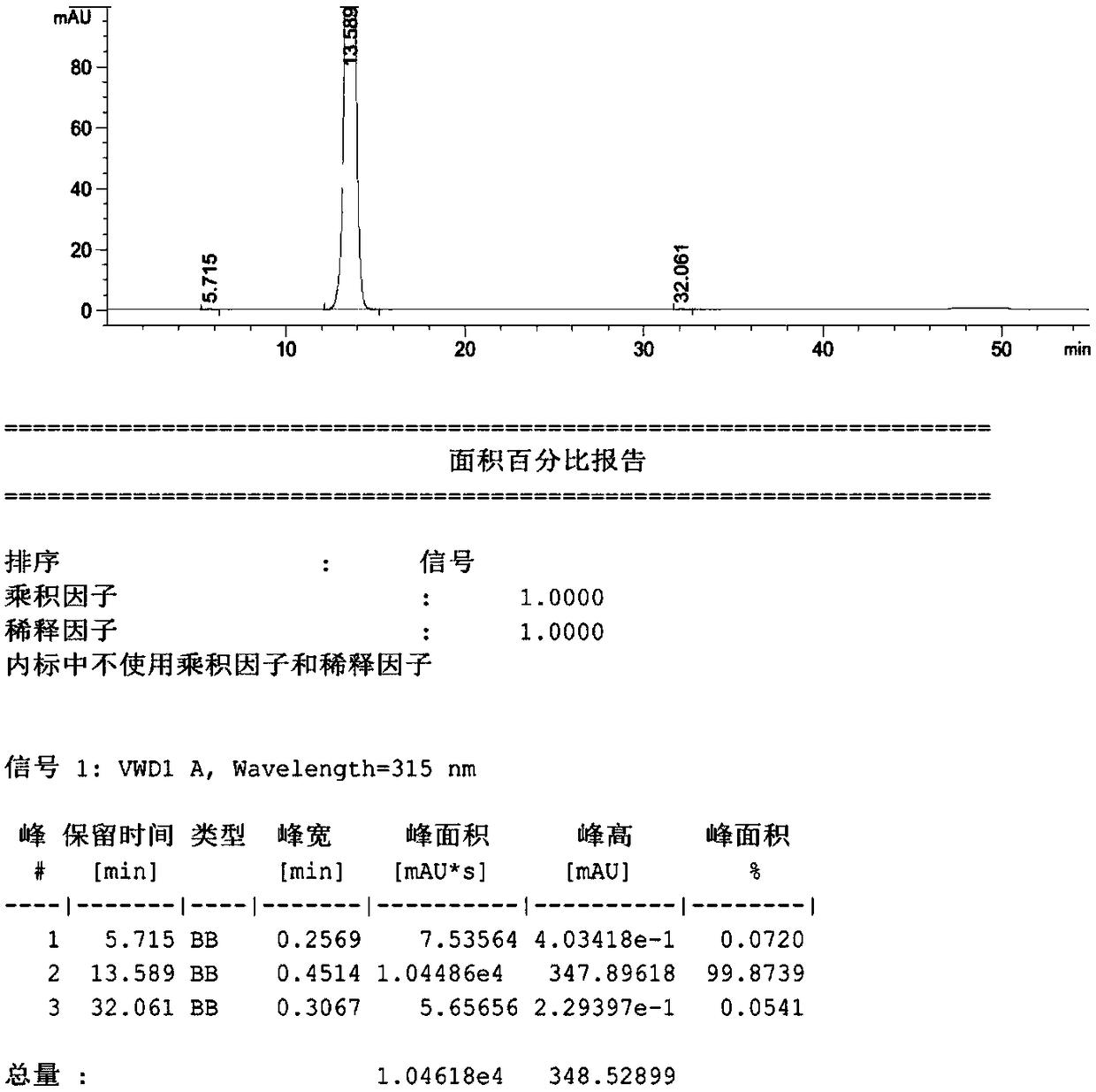
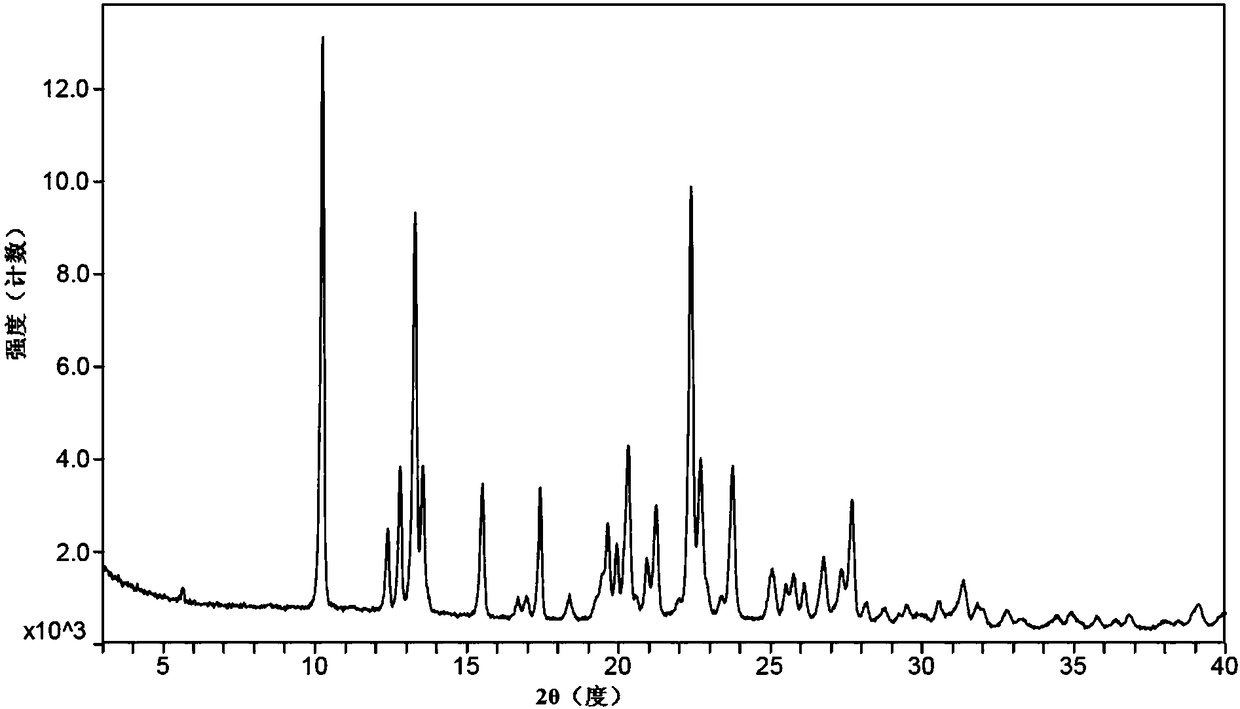
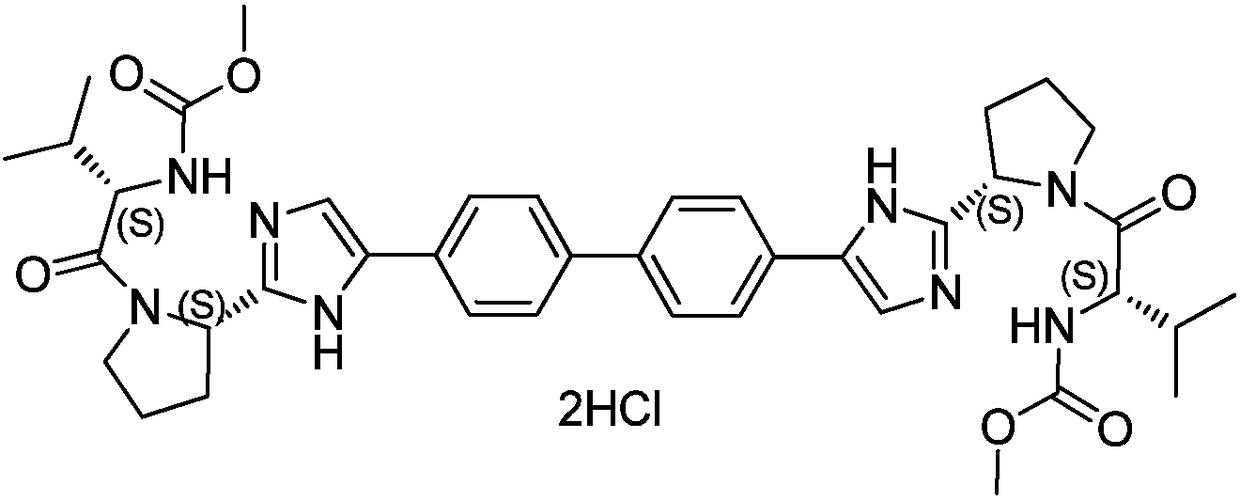
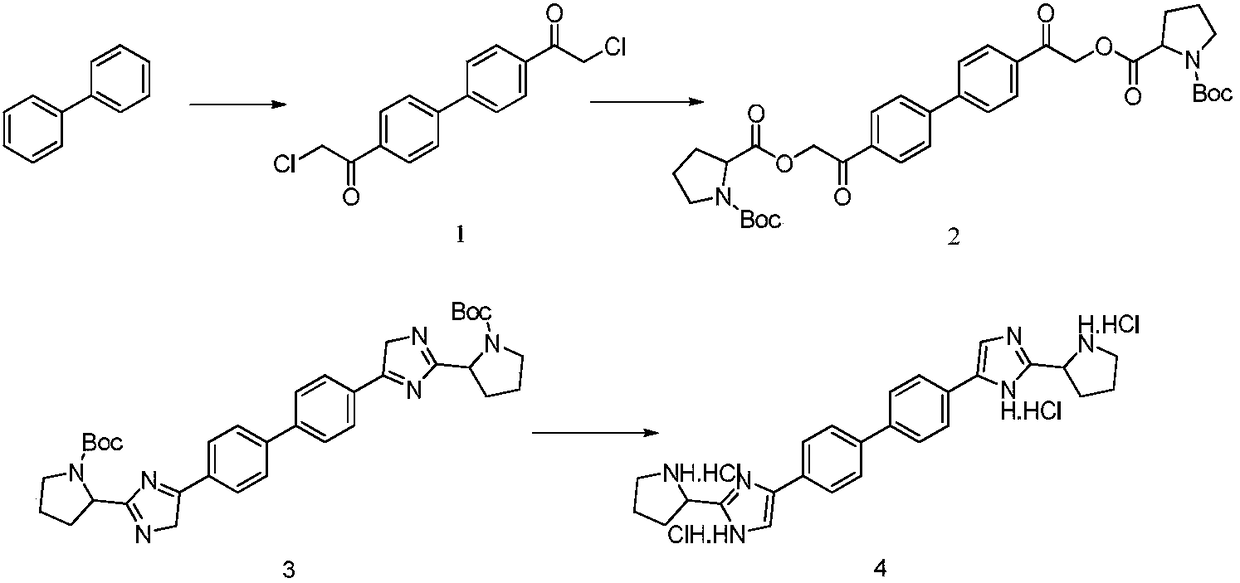
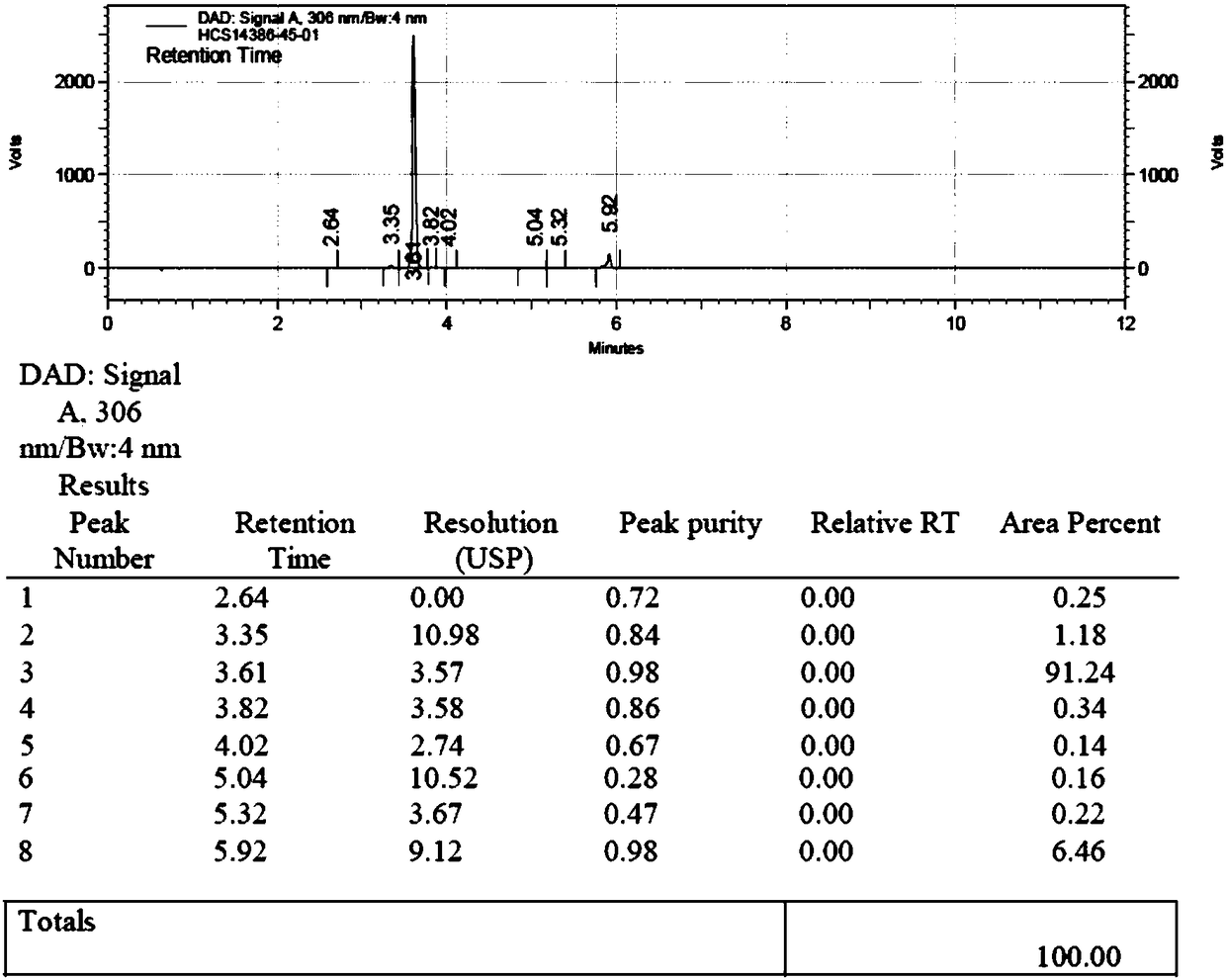
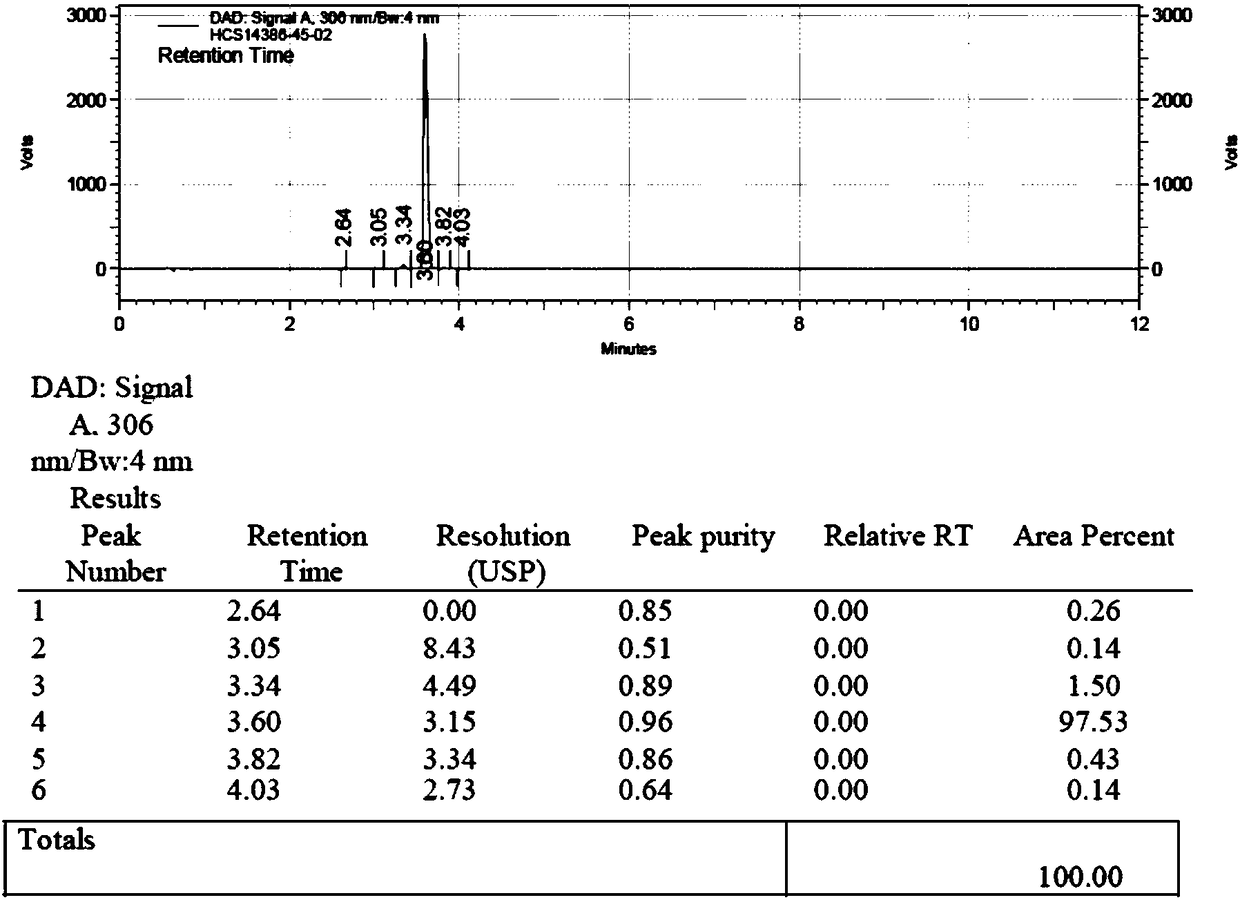
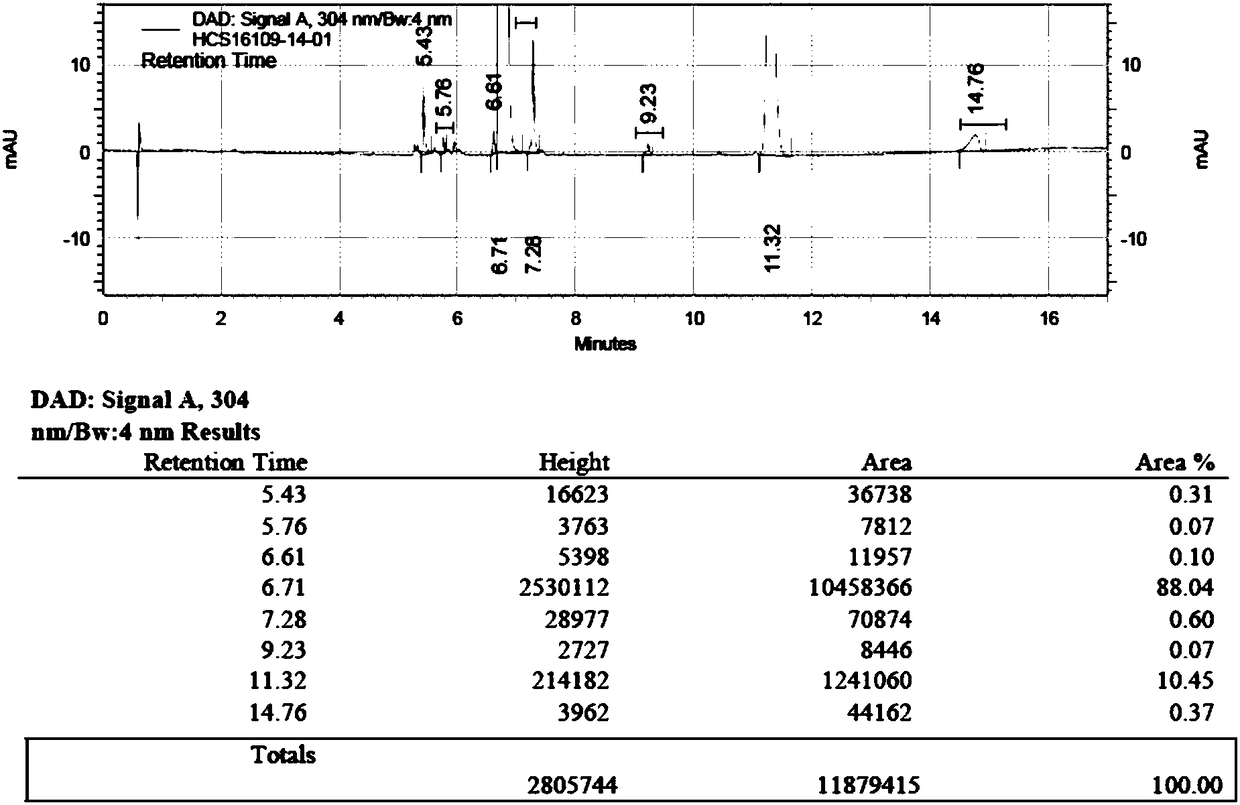
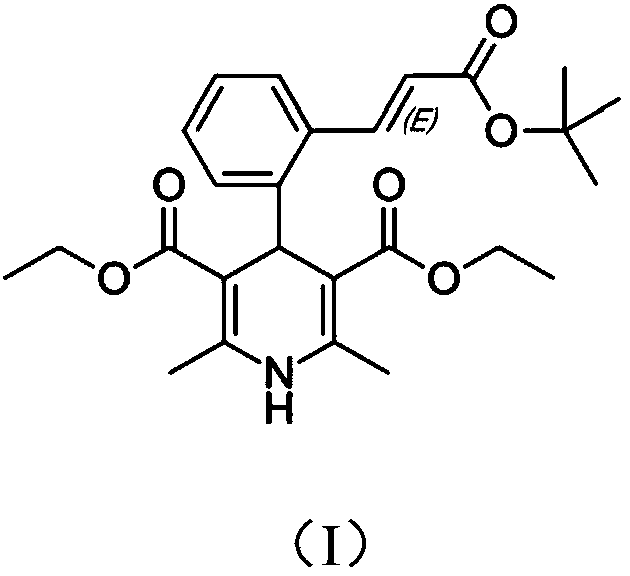
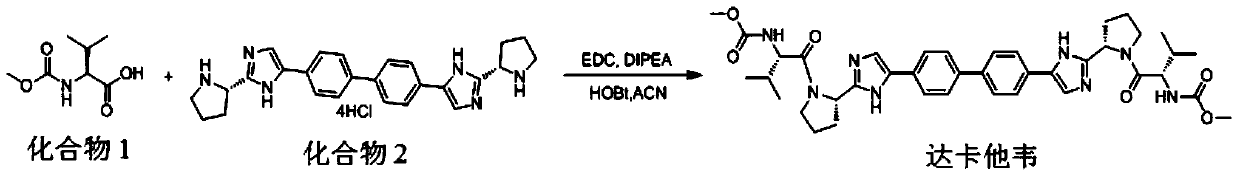
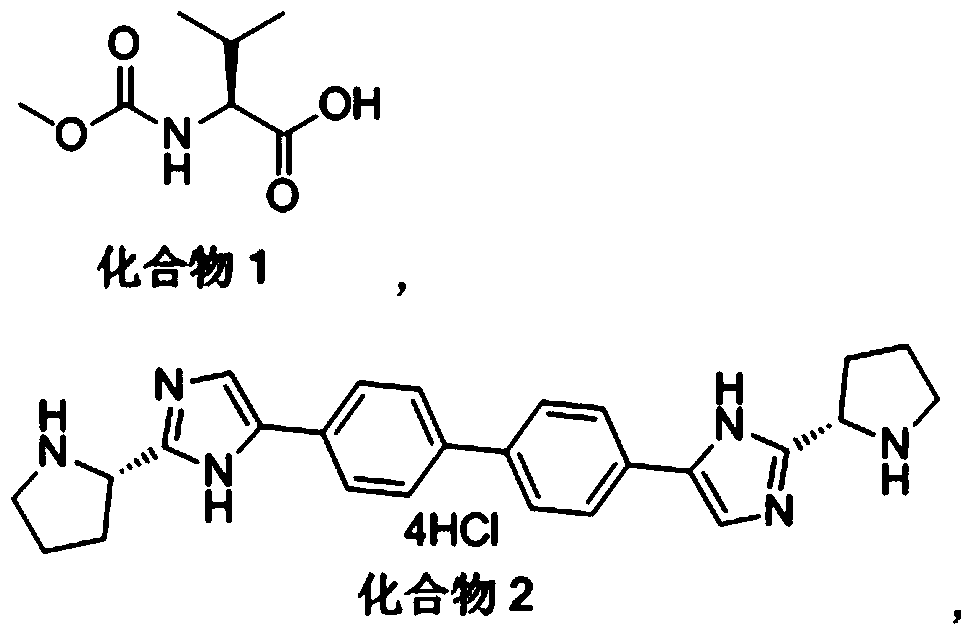
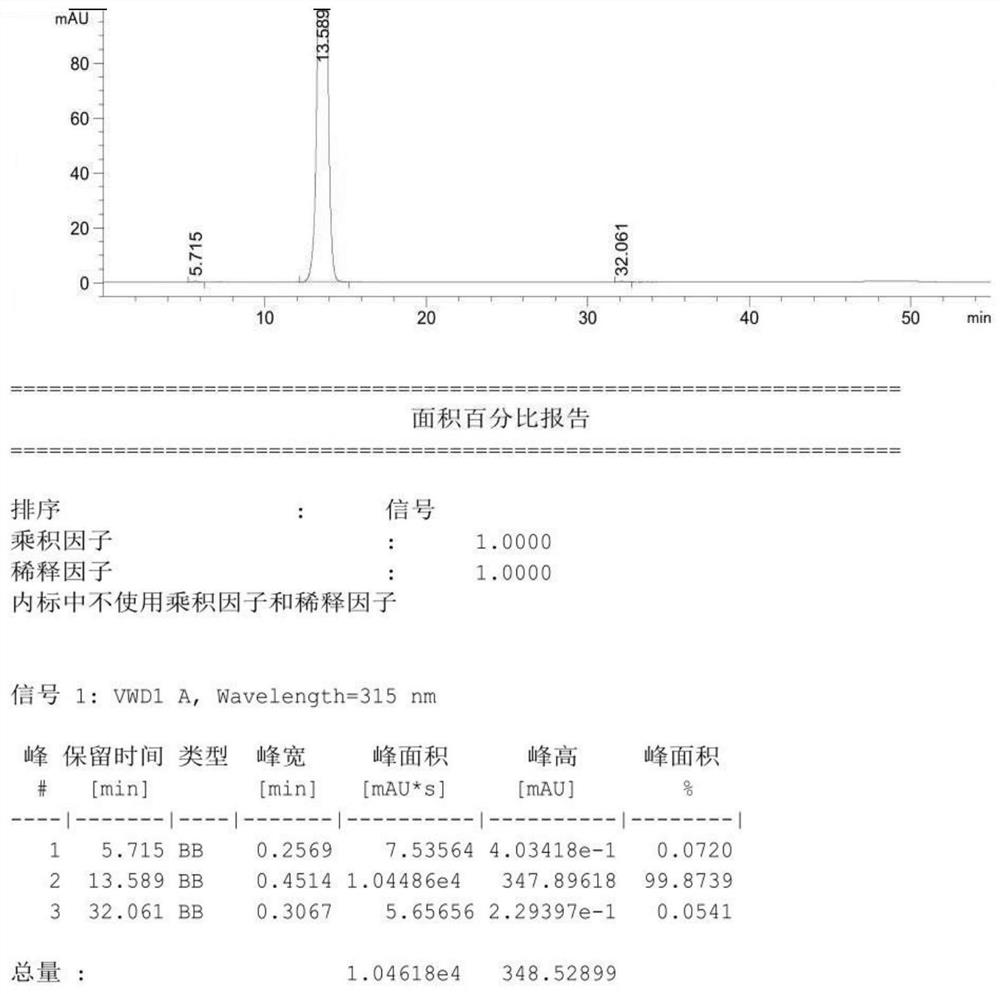
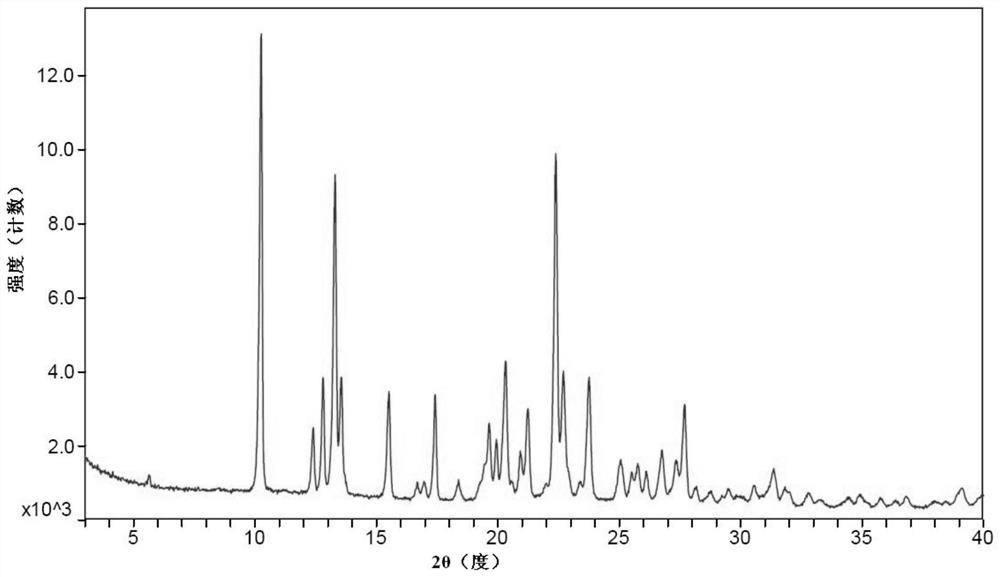
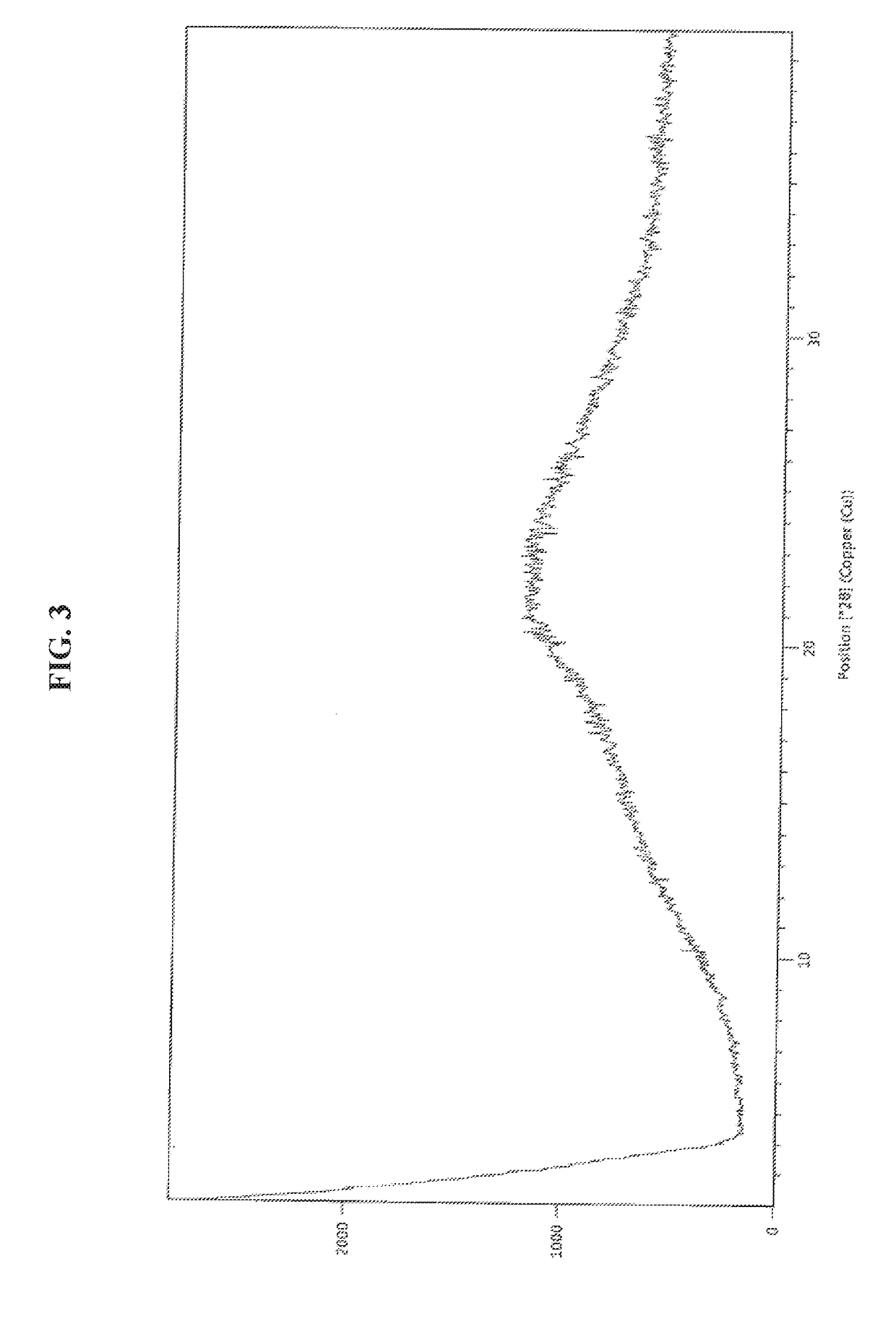
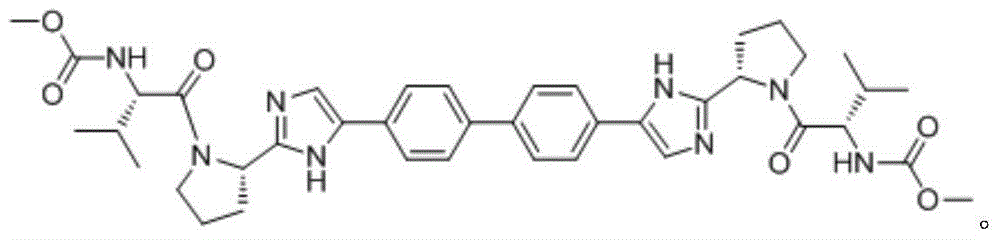
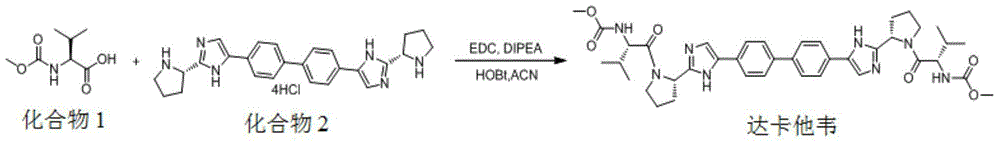
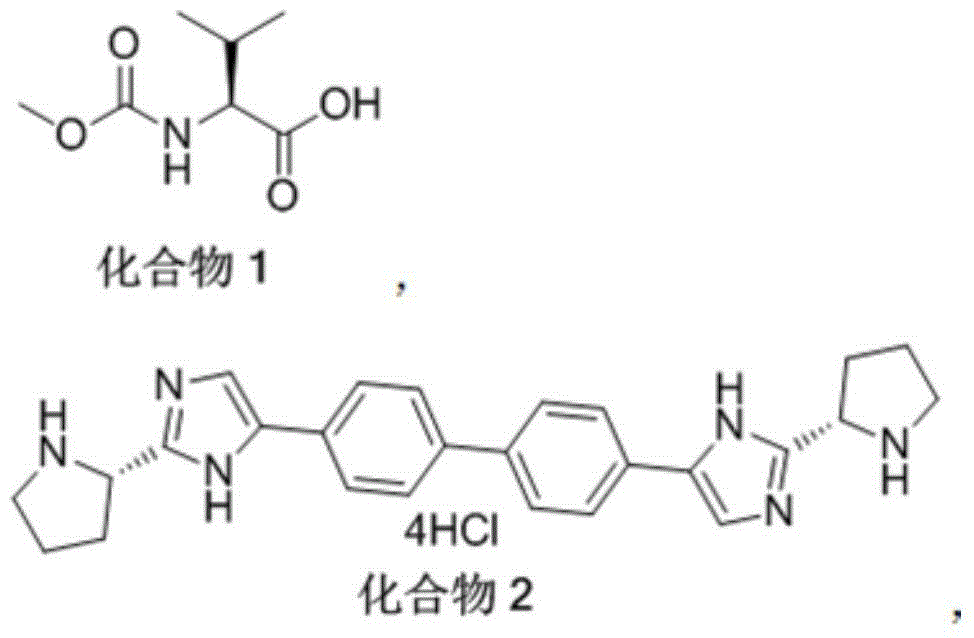
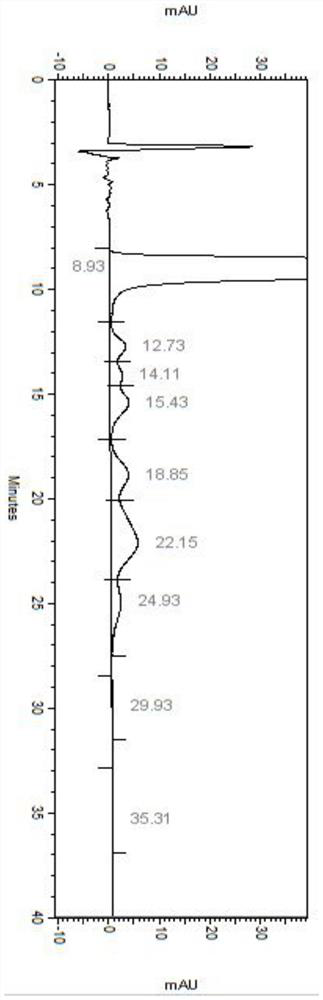
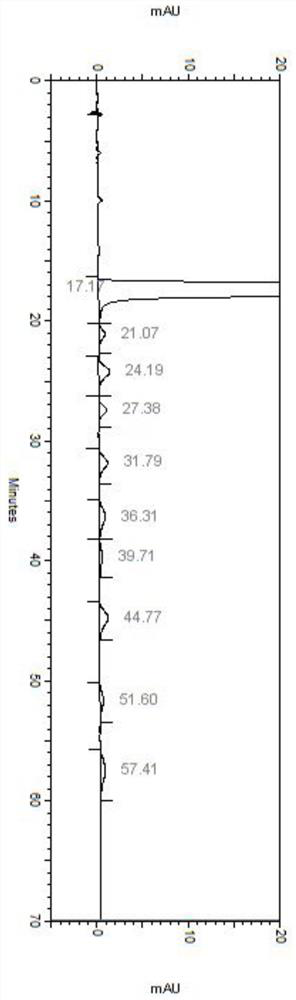
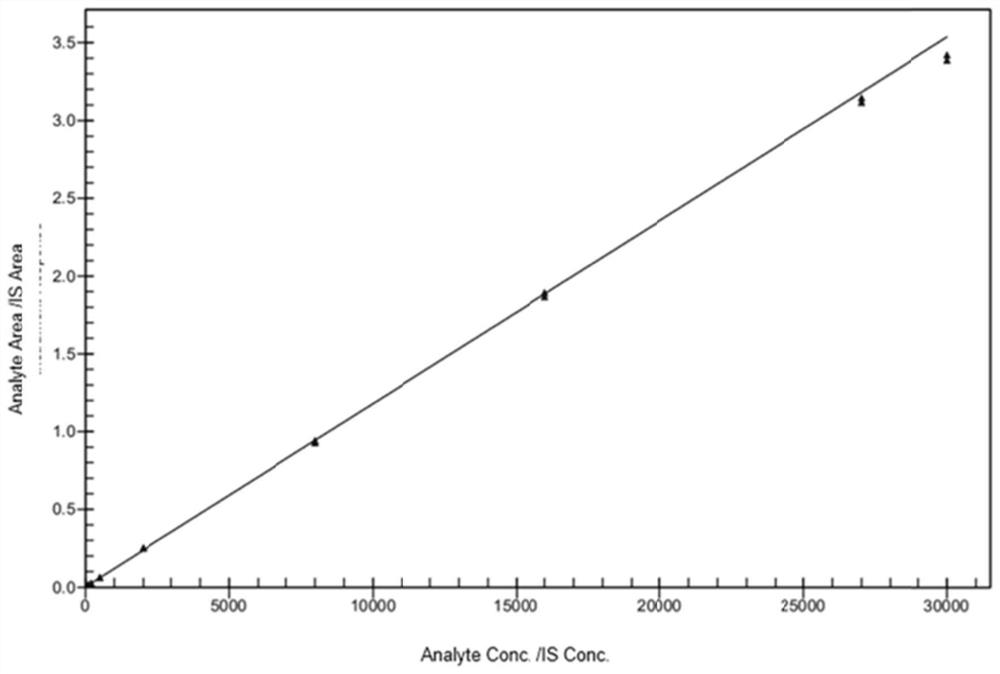
Patents
Literature
30 results about "Daclatasvir" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
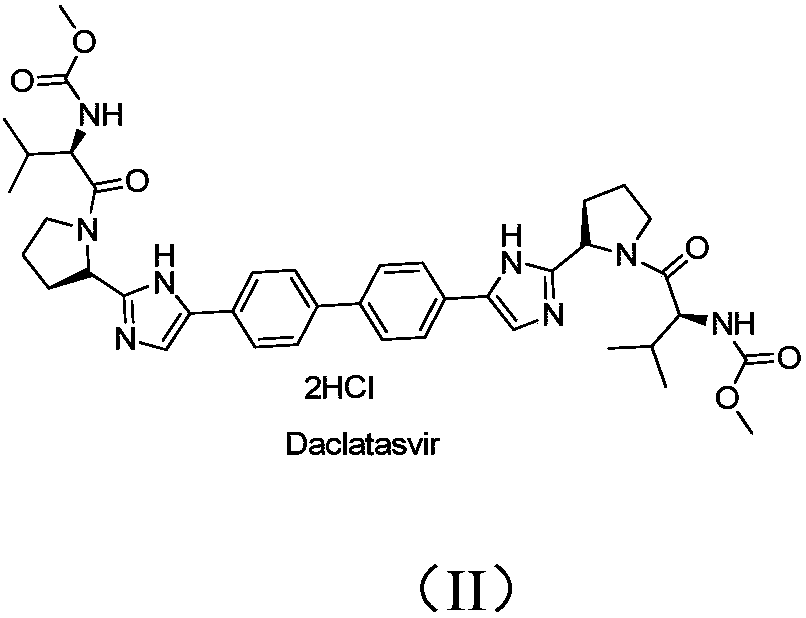
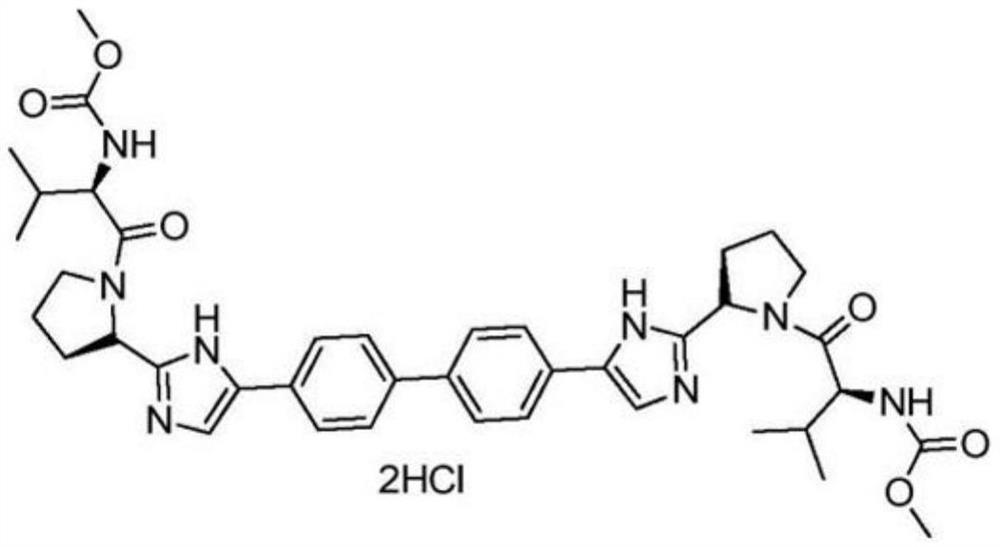
Daclatasvir is used with another antiviral medication (sofosbuvir) to treat chronic (long-lasting) hepatitis C, a viral infection of the liver. Daclatasvir should never be used without sofosbuvir. Daclatasvir and sofosbuvir may also be used with another antiviral medication (ribavirin).
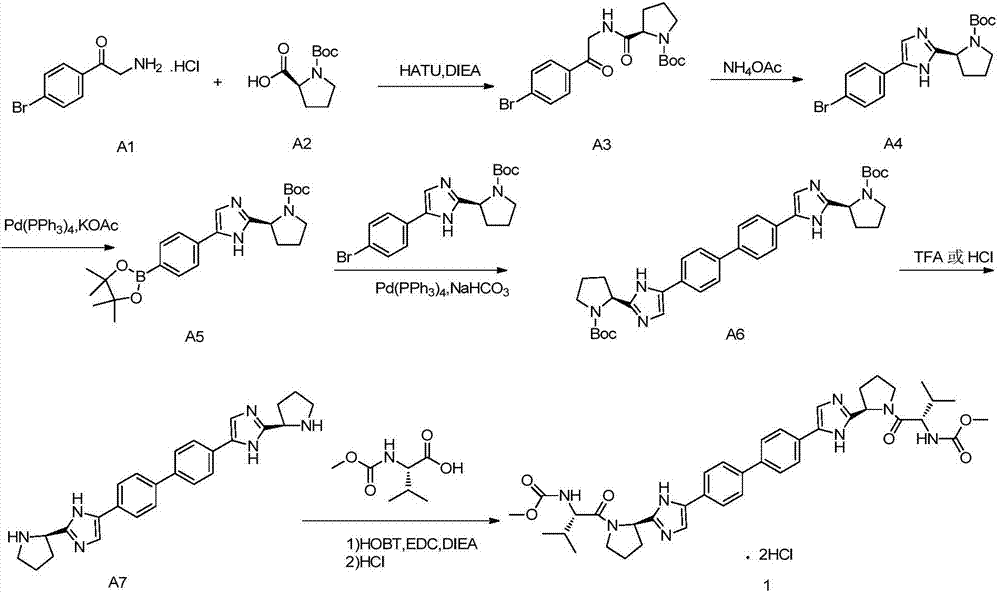
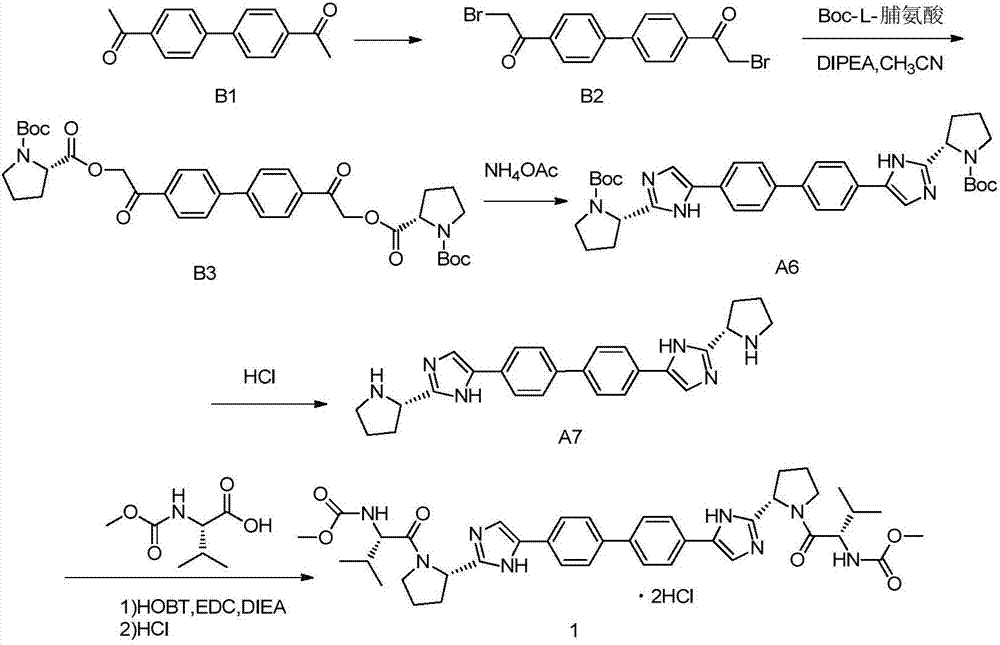
Daclatasvir synthetic method
InactiveCN106256825AHigh purityThe synthesis process is simpleOrganic chemistryBenzeneOrganic solvent
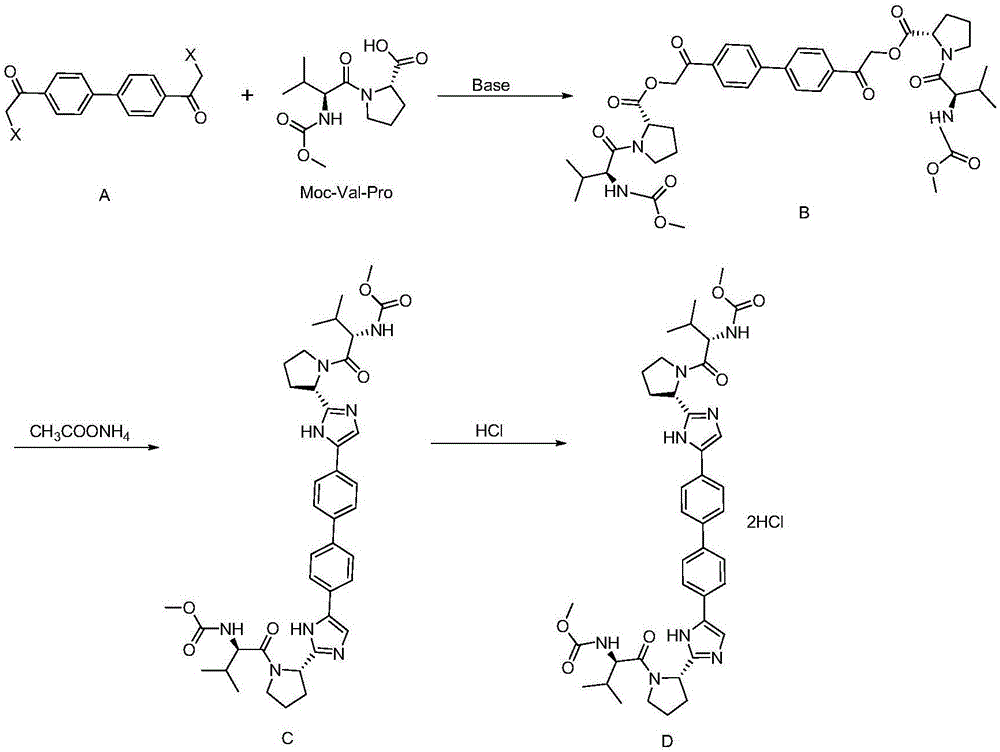
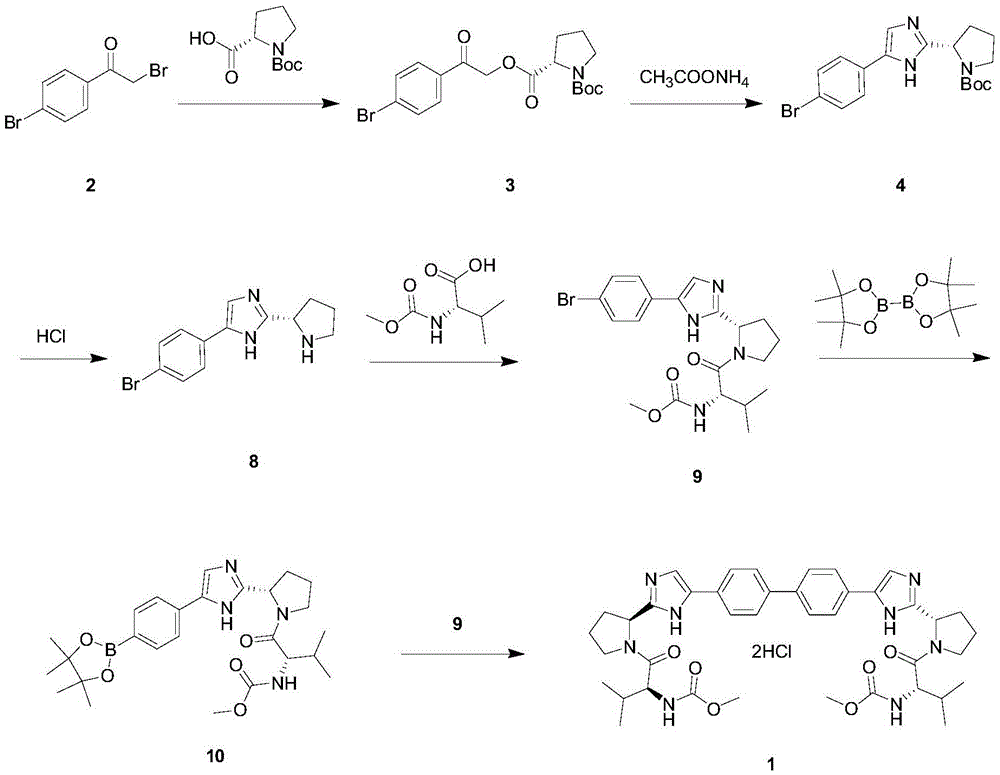
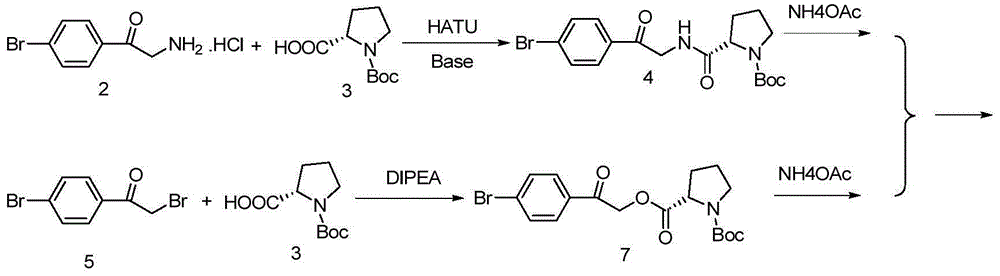
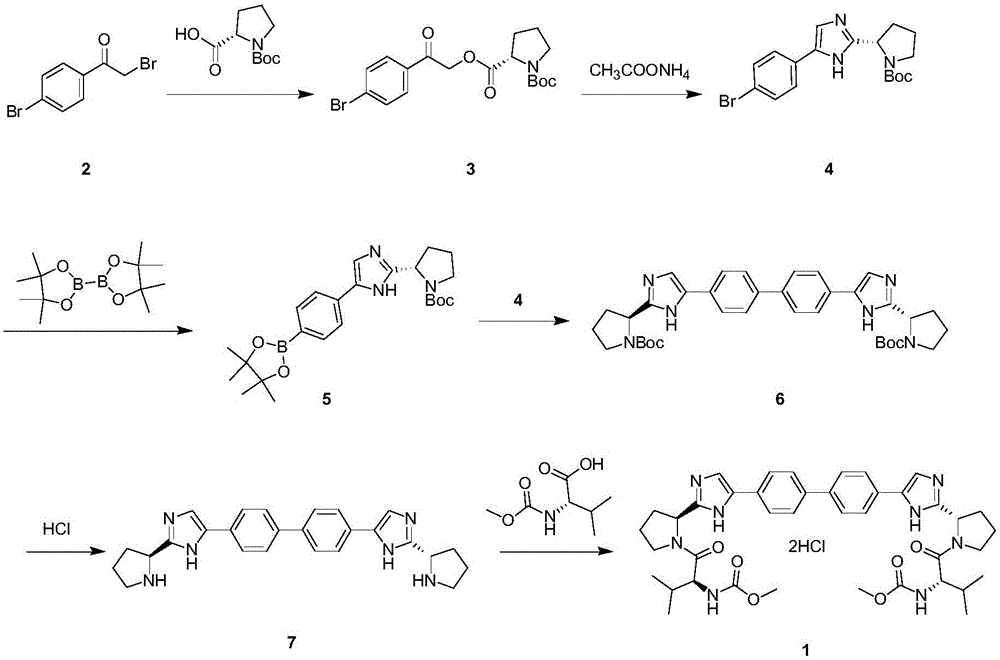
The invention provides a daclatasvir synthetic method. The method comprises the following steps: taking 4,4'-di(2-halogenated acetyl)biphenyl as a raw material, performing an esterification reaction with N-(methoxycarbonyl)-L-valine-L-proline in an organic solvent under alkali existence to obtain an intermediate B; then performing a dehydrating-cyclizing reaction on the intermediate B and ammonium acetate in an xylene solution at the temperature of 100-120 DEG C to obtain free alkali C; and reacting the free alkali C and HCl to obtain a daclatasvir crude product; and finally re-crystallizing the daclatasvir crude product to obtain daclatasvir. The synthetic method has the advantages of simple synthesis route, convenient operation and simple purifying, the purity of daclatasvir obtained by the synthesis reaction is high, the yield is large, quality of the daclatasvir bulk drug is greatly increased, production cost is reduced, and the method is suitable for large industrial production.
Owner:SICHUAN TONGSHENG BIOTECH
Novel method for synthesizing anti-hepatitis C virus novel medicine daclatasvir
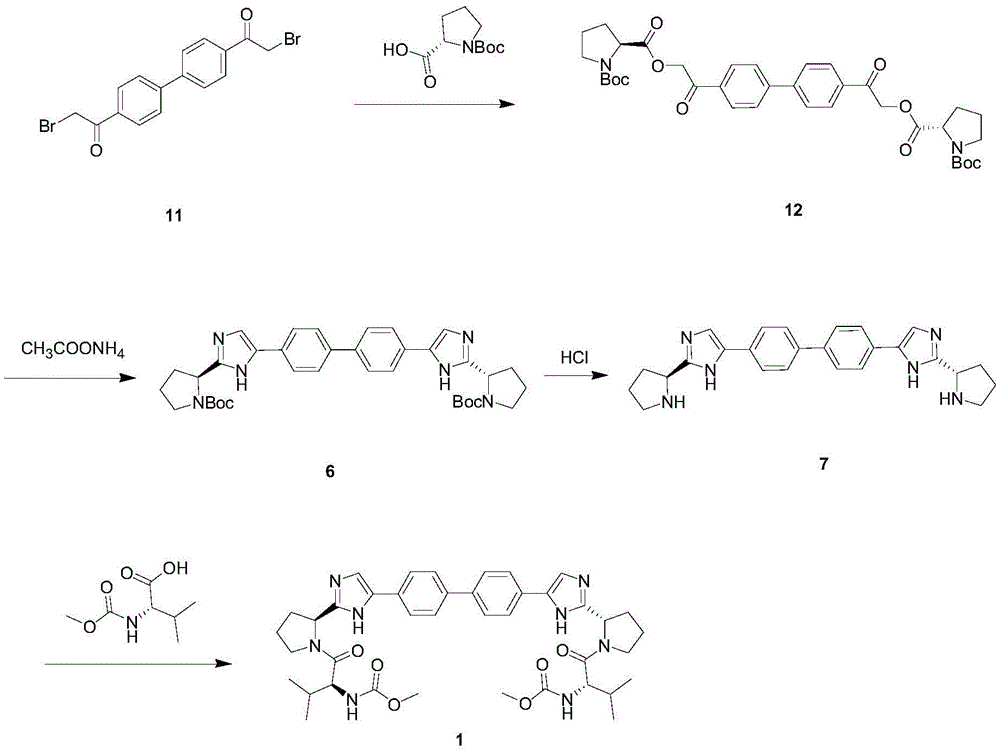
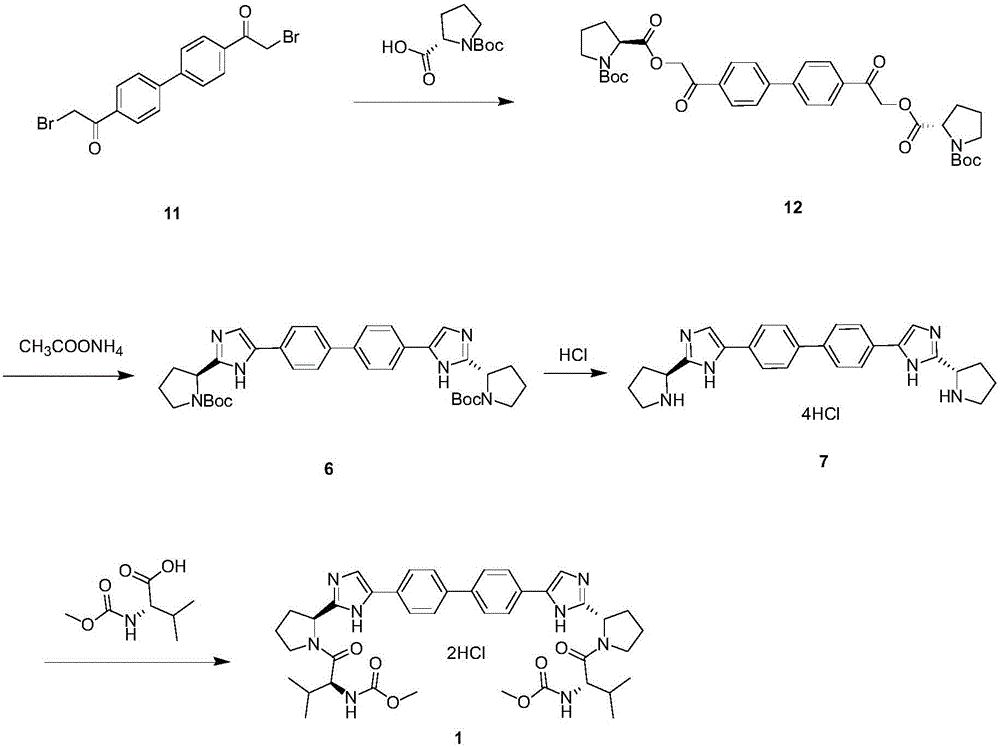
The invention provides a novel method for synthesizing anti-hepatitis C virus novel medicine daclatasvir. 4,4'-di(2-bromoacetyl) biphenyl serve as a raw material and first undergoes the condensation reaction with N-(methoxycarbonyl)-L-valyl-L-proline to obtain 4,4'-di(N-(methoxycarbonyl)-L-valyl-L-proline ester acetyl) biphenyl, and 4,4'-di(N-(methoxycarbonyl)-L-valyl-L-proline ester acetyl) biphenyl undergoes ring closing reaction with ammonium acetate to synthesize hepatitis C virus (HCV) NS5A inhibitor daclatasvir through two-step reaction. The novel method for synthesizing anti-hepatitis C virus novel medicine daclatasvir has the advantages that the reaction and separation and purification steps are fewer, the synthetic cycle is short and the production cost is low; and the method has wide prospect of large scale industrial application.
Owner:上海步越化工科技有限公司
Novel method for synthesizing daclatasvir intermediate
InactiveCN105153128AGet rid of the useLow costCarbonyl compound preparation by condensationHalogenDaclatasvir
The invention provides a novel method for synthesizing a daclatasvir intermediate, particularly a preparation method of a compound disclosed as Formula 1. The method uses biphenyl and halogen acyl bromide as initial raw materials, and avoids using high-cost reaction raw materials. Thus, the preparation method is simple and safe, and is suitable for industrial production.
Owner:SHANGHAI FOREFRONT PHARMA CO LTD
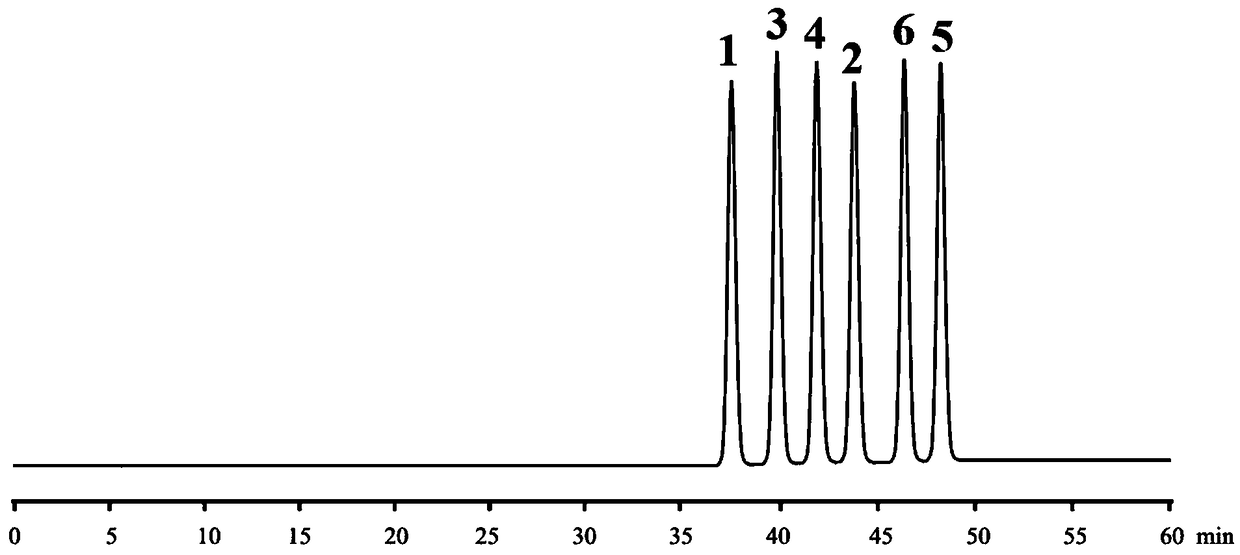
Method for separating and detecting daclatasvir hydrochloride and optical isomers thereof
ActiveCN108732280AQuality improvementEfficient separationComponent separationPotassium hexafluorophosphateCellulose
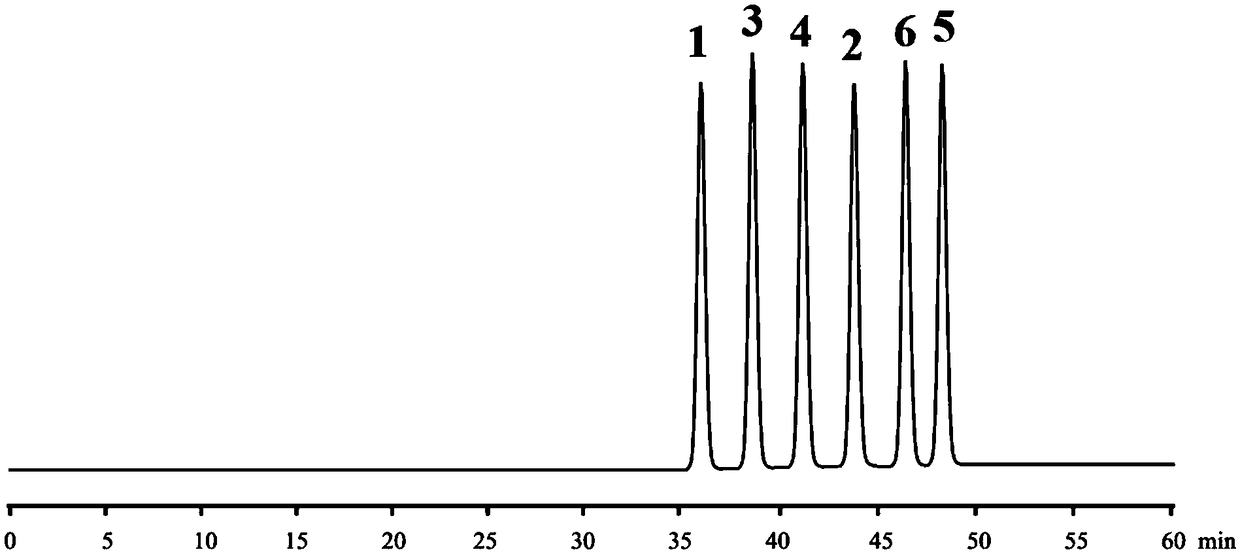
The invention in particular relates to a method for separating and detecting daclatasvir hydrochloride and optical isomers thereof. The method for separating and determining daclatasvir hydrochlorideand optical isomers thereof (impurities) by using liquid chromatography is characterized by comprising the following steps: adopting a chiral chromatographic column taking cellulose tris(3,5-dimethylphenylcarbamate) as a filler, and taking a mixed solution which takes sodium hexafiuorophosphate, potassium hexafluorophosphate, formic acid, acetic acid, phosphoric acid or aqueous phosphate solutionas an aqueous phase and takes acetonitrile or methanol as an organic phase as a mobile phase. According to the separating and detecting method disclosed by the invention, the daclatasvir hydrochlorideand optical isomers thereof (impurities) can be effectively separated, the degree of separation reaches 3.0 or higher, and complete baseline separation is realized, so that the quality of the daclatasvir hydrochloride can be accurately and effectively controlled. With the adoption of the separating method disclosed by the invention, the time of separating and detecting the daclatasvir hydrochloride and optical isomers thereof is within 30-80 minutes. The method disclosed by the invention has the advantages of being simple, rapid, accurate and the like.
Owner:SUNSHINE LAKE PHARM CO LTD
Anti-hepatitis C Daclatasvir synthesis method
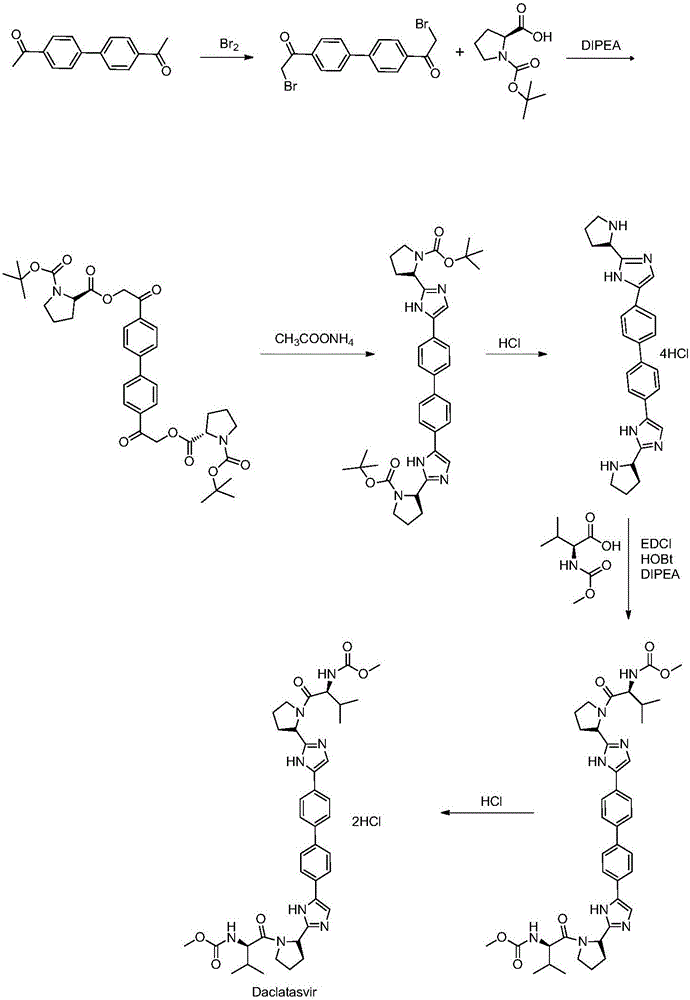
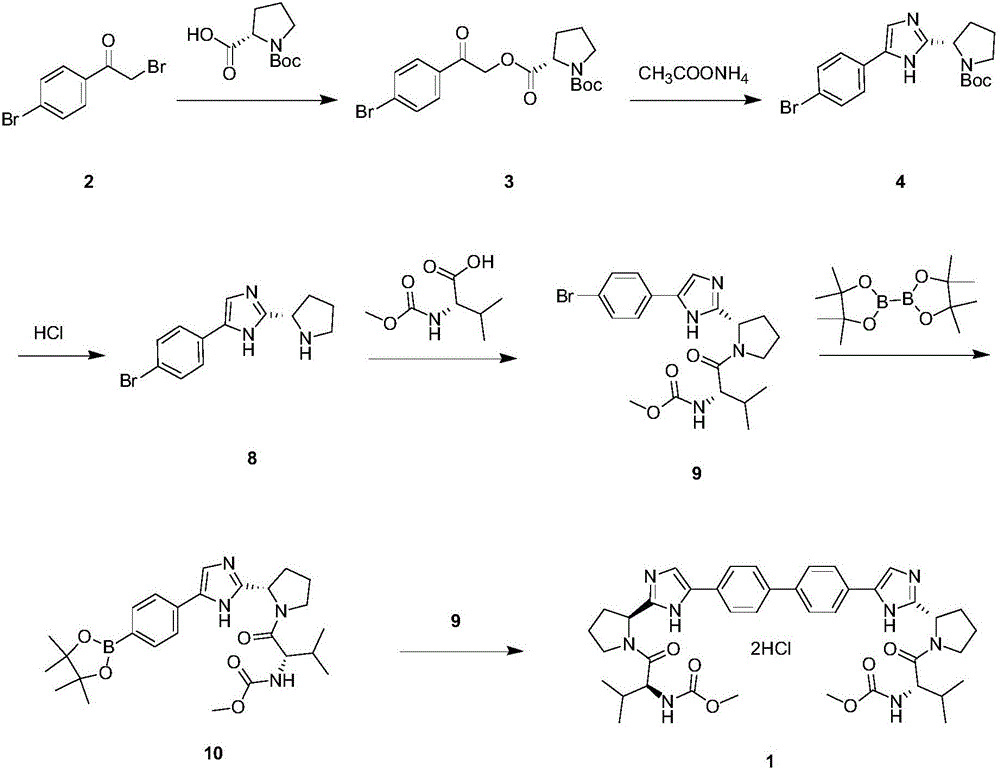
The invention discloses an anti-hepatitis C Daclatasvir synthesis method. The anti-hepatitis C Daclatasvir synthesis method comprises the following steps that 1, 4,4'-di(2-chloracetyl) biphenyl and Boc-L-proline perform condensation to generate ester; 2, the ester and ammonium acetate perform ring closure to obtain imidazole; 3, Boc is removed from the imidazole to obtain hydrochloride; 4, the hydrochloride is taken for Moc-L-valine condensation to obtain Daclatasvir, wherein in the step 1, a solvent is acetonitrile, and an acid-binding agent is diisopropylethylamine. By the adoption of the anti-hepatitis C Daclatasvir synthesis method, the preparation raw materials are low in cost, synthetic route steps are few, reaction is simple, and popularization and application are promoted.
Owner:上海步越化工科技有限公司
Compound and applications of compound in preparation of anti-hepatitis C virus drugs
ActiveCN106946775ARich diversityEasy to separate and purifyOrganic chemistryAntiviralsAnti virusBoceprevir
The present invention discloses a compound and applications of the compound in preparation of anti-hepatitis C virus drugs, wherein the structure formula of the compound is represented by a formula I or II, and the compound represented by the formula I or compound represented by the formula II can be subjected to drug combination with other anti-virus drugs such as interferon (PEG IFN-[alpha]), ribavirin (RBV), boceprevir, telaprevir, simeprevir, sofosbuvir, daclatasvir and the like t prepare anti-HCV products and other anti-virus infection products. According to the present invention, the compound has rich functional group diversity and modificability, and the product is relatively easy to separate and purify; the compounds can well inhibit HCV and other viruses, are obtained through phenotype screening, have different antiviral mechanisms, have extremely novel and innovated structures in the anti-virus field, and are not reported in the prior art; and the compound of the present invention has broad development and application prospect.
Owner:TSINGHUA UNIV
Daclatasvir film coating tablet preparation and preparation method thereof
InactiveCN104546780AImprove liquidityGood compressibilityOrganic active ingredientsDigestive systemPolythylene glycolStearic acid
The invention discloses a daclatasvir film coating tablet preparation. The preparation is prepared from the following active ingredients in percentage by mass: 12.0-20.0 percent of daclatasvir, 2.0-8.0 percent of a disintegrating agent, 50.0-80.0 percent of a diluting agent and 0.5-1.5 percent of a lubricating agent, wherein the disintegrating agent is one or more of croscarmellose sodium, hydroxypropyl methylcellulose and sodium carboxymethyl starch, one or two of sodium carboxymethylcellulose and hydroxypropyl methylcellulose as first choice, and preferably sodium carboxymethylcellulose; the diluting agent is one or more of microcrystalline cellulose, lactose anhydrous, mannitol and polyethylene glycol, one or two of microcrystalline cellulose and mannitol as first choice, and preferably microcrystalline cellulose; and the lubricating agent is one or more of magnesium stearate, talcum powder, aerosil and calcium stearate, one or two of magnesium stearate and aerosil, and preferably magnesium stearate. The daclatasvir film coating tablet preparation has the advantages of simple process, high yield, good stability and the like, and is easy for large-scale industrial production.
Owner:ANHUI YELLEN PHARMA
Preparation method of deuterated daclatasvir intermediate
PendingCN112939814AIncrease profitReduce pollutionCarbamic acid derivatives preparationOrganic compound preparationCombinatorial chemistryValine
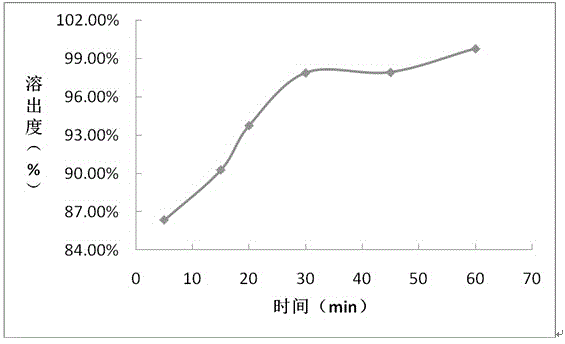
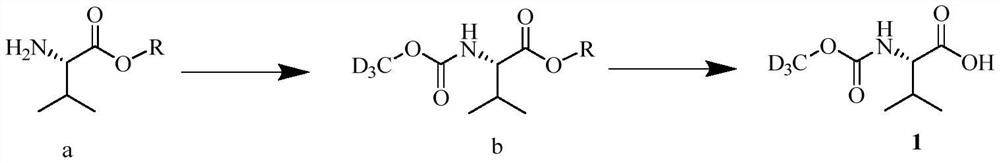
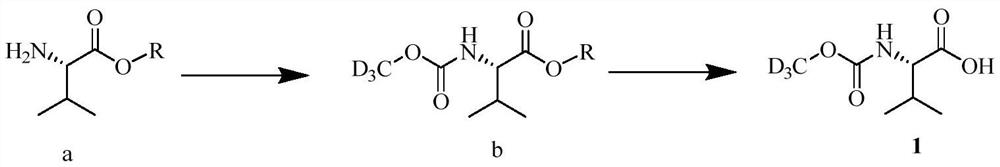
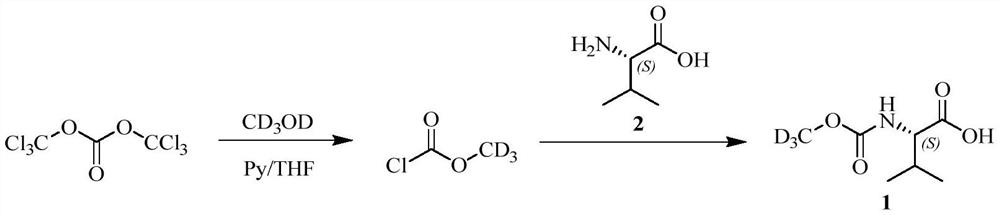
The invention provides a preparation method of a deuterated daclatasvir intermediate, and particularly relates to a preparation method of N-(trideuteromethoxycarbonyl)-L-valine. According to the preparation method, the intermediate is obtained through two-step synthesis by taking CDI as an activating reagent. Compared with a triphosgene route in the prior art, the route has the advantages that the yield and the utilization rate of deuterated methanol can be improved, and meanwhile, the pollution to the environment is greatly reduced.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
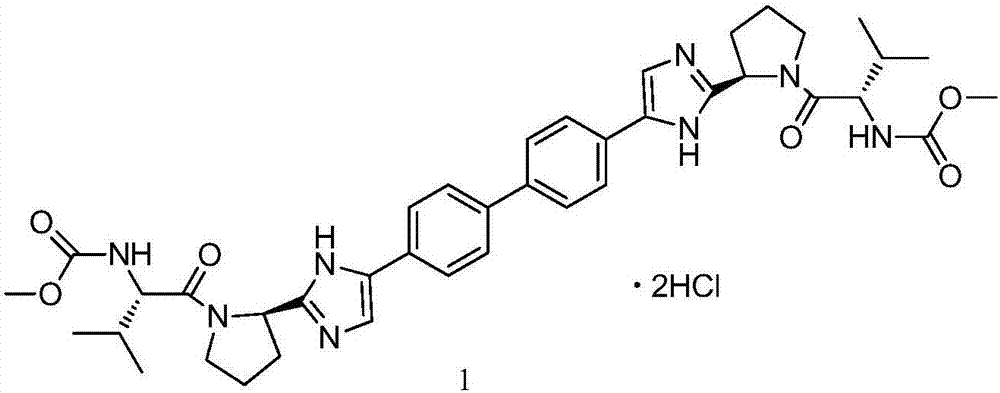
Amorphous forms of daclatasvir dihydrochloride
InactiveUS20160194352A1Fast decaySustained efficacyBiocidePeptide/protein ingredientsDaclatasvirStereochemistry
The present application relates to the amorphous form of Daclatasvir dihydrochloride and the processes for the preparation thereof. The application further provides its solid dispersion having Daclatasvir dihydrochloride in amorphous form and process for its preparation.
Owner:DR REDDYS LAB LTD
Refining method of daclatasvir hydrochloride
The invention discloses a refining method of daclatasvir hydrochloride. The refining method comprises the following steps: (1) adding a daclatasvir hydrochloride crude product into methanol and dissolving at 55 to 65 DEG C; (2) adding activated carbon and de-coloring at 55 to 65 DEG C; (3) filtering and raising the temperature of filtrate to 55 to 65 DEG C; dropwise adding a mixed solvent of a water-soluble aprotic solvent A and a proton solvent B and crystallizing; after dropwise adding, and cooling to 20 to 30 DEG C, stirring and crystallizing for 2 h; (4) filtering a mixture obtained by stirring and crystallizing in step (3); drying a filter cake in vacuum to obtain the high-purity daclatasvir hydrochloride. The method disclosed by the invention can be used for effectively removing impurities in the daclatasvir hydrochloride crude product and the purity of a product reaches 99.5 percent or more; the content of isomer impurities and other single impurities is not greater than 0.1 percent and the refining yield is not lower than 85 percent; furthermore, the refining method disclosed by the invention has a simple technological process and industrial production is easy to realize bythe technology.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Industrial production method for Daclatasvir key intermediate
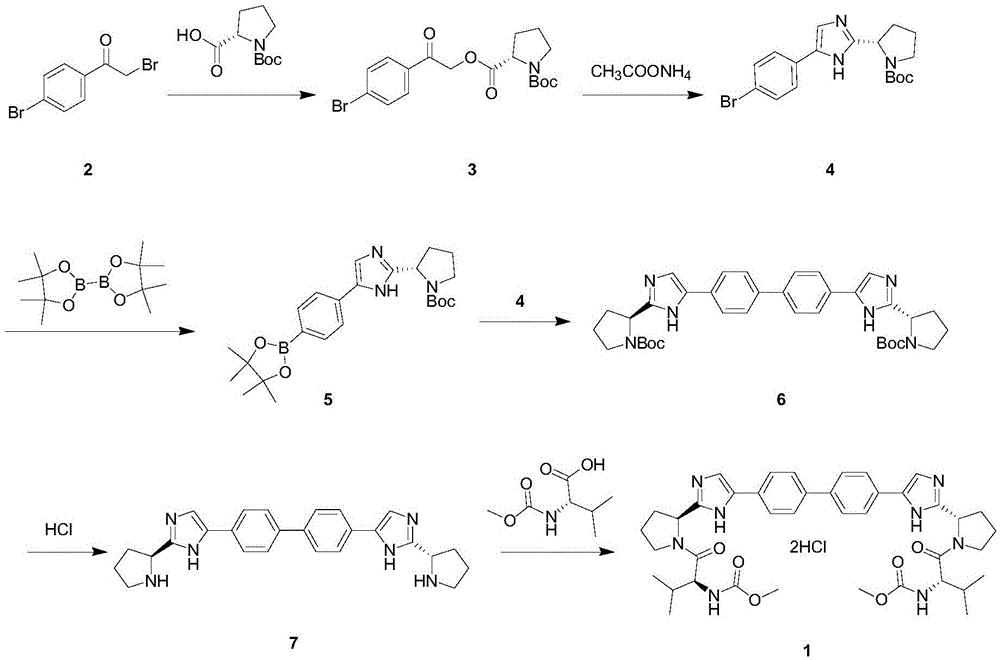
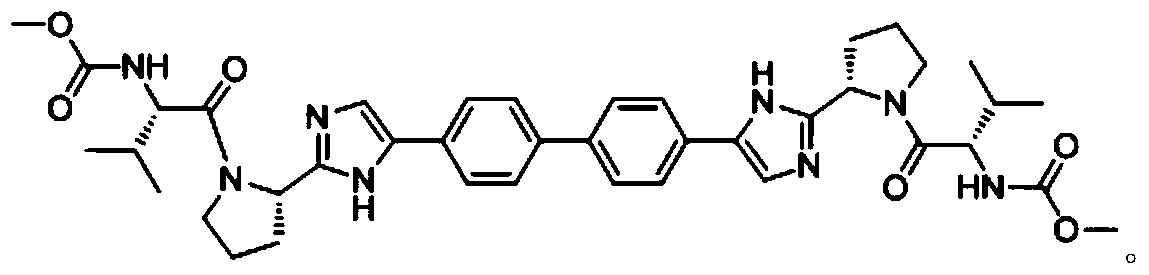
The invention relates to the technical field of biochemical engineering and specifically relates to an industrial production method for a Daclatasvir key intermediate 5,5'-[1,1'-biphenyl]-4,4'-diyl di[2-(2S)-2-pyrrolidyl-1H-imidazole]terthydrochloride. The 5,5'-[1,1'-biphenyl]-4,4'-diyl di[2-(2S)-2-pyrrolidyl-1H-imidazole]terthydrochloride is prepared according to a one-pot method. Compared with aconventional process, the industrial production method for the Daclatasvir key intermediate 5,5'-[1,1'-biphenyl]-4,4'-diyl di[2-(2S)-2-pyrrolidyl-1H-imidazole]terthydrochloride is capable of efficiently and stably producing high-quality 5,5'-[1,1'-biphenyl]-4,4'-diyl di[2-(2S)-2-pyrrolidyl-1H-imidazole]terthydrochloride, the purity of the intermediate is greater than 99%, and the content of unknown single impurities is smaller than 0.3%.
Owner:GENCHEM & GENPHARM CHANGZHOU CO LTD
Method for preparing daclatasvir
InactiveCN108069941ASuitable for industrialized mass productionHigh purityOrganic chemistryChemical industryOrganic solvent
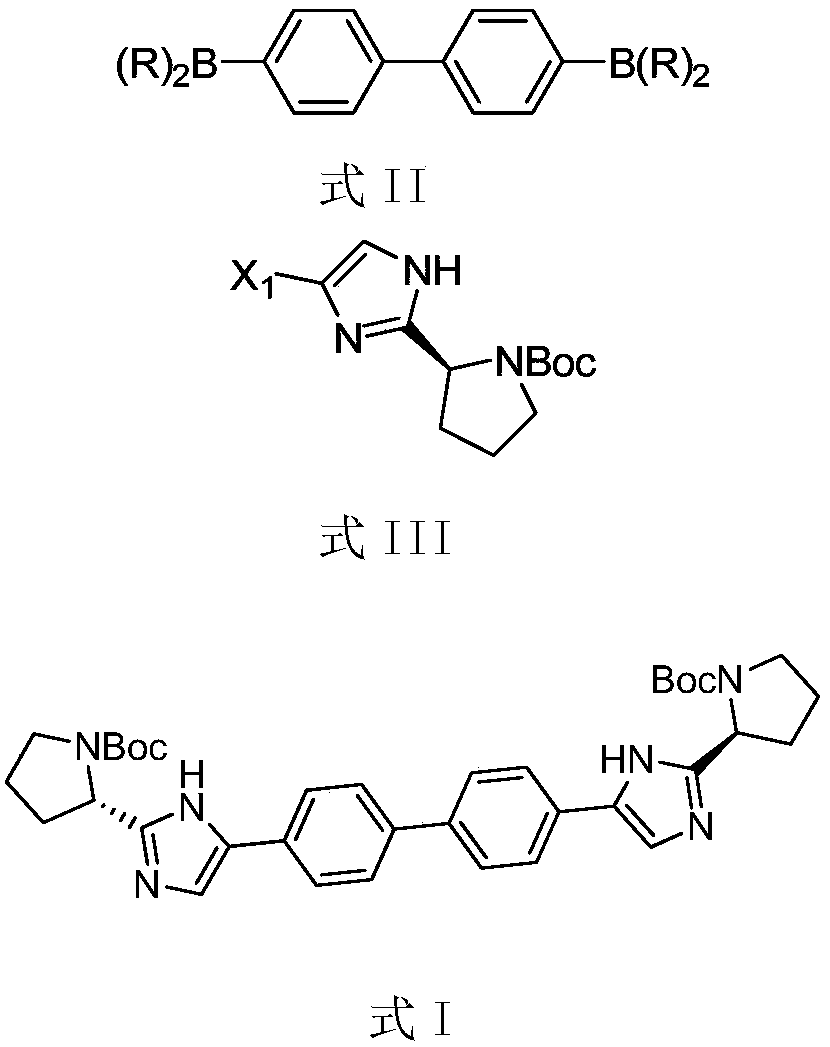
The invention provides a method for preparing daclatasvir and belongs to the field of pharmaceutical and chemical industry. According to the method, a compound shown in a formula II and a compound shown in a formula III are subjected to a condensation reaction, a mixture A is obtained, alkali is added, the mixture is stirred at a certain temperature, so that the mixture A reacts, an organic solvent is added for extraction and liquid separation after the reaction ends, an organic phase is concentrated and dried, and daclatasvir is obtained. The method has the characteristics that the product produced with the method has high purity, the method is high in yield, low in cost and simple to operate and the process is stable.
Owner:SUNSHINE LAKE PHARM CO LTD
Composition and application thereof to prevention and control avian infectious laryngotracheitis
InactiveCN108619143AAvoid spreadingReduce mortalityOrganic active ingredientsAnimal feeding stuffInfectious laryngotracheitisActive component
The invention provides a composition and application thereof to prevention and control avian infectious laryngotracheitis and relates to the field of veterinary drugs. The composition is prepared fromlacidipine and daclatasvir. According to the composition, two active components including the lacidipine and the daclatasvir are compounded, so that the composition has a strong killing effect on avian infectious laryngotracheitis viruses, and can be used for inhibiting the spreading of the avian infectious laryngotracheitis viruses and effectively reducing the death rate of infected poultry; thedrug resistance is not easy to generate; the composition has the characteristics of rapid effect and excellent curative effect.
Owner:绵阳市农业科学研究院
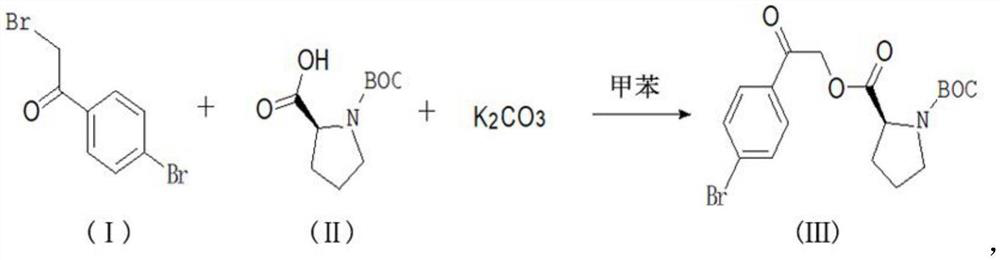
A kind of synthetic method of daclatasvir starting material
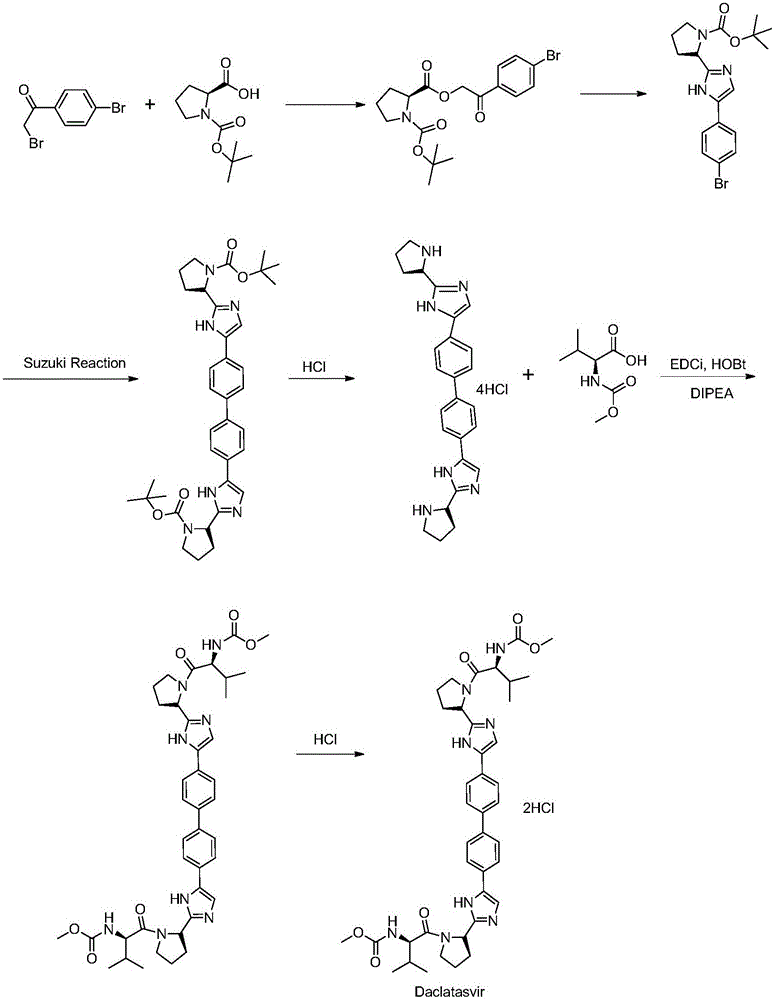
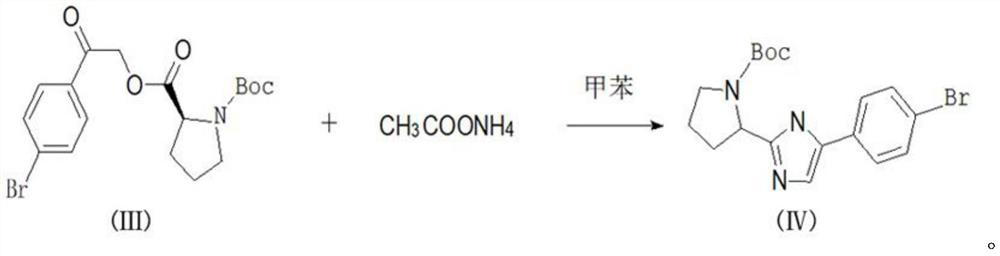
The present invention relates to a kind of synthetic method of daclatasvir starting material, 2,4'-dibromoacetophenone shown in formula I, L-BOC-proline shown in formula II, potassium carbonate and Toluene is reacted to form an intermediate product shown in formula III, and the intermediate product shown in formula III is reacted with ammonium acetate to form a compound shown in formula IV. The method for synthesizing the daclatasvir starting material of the invention has simple reaction route and low cost of raw materials, and is suitable for industrial production.
Owner:CHANGZHOU YINSHENG PHARMA
Synthetic method of daclatasvir
InactiveCN107501243ALow costHigh yieldOrganic chemistryTert-Butyloxycarbonyl protecting groupDaclatasvir
The invention discloses a synthetic method of daclatasvir. The method adopts bis(2-bromoacetyl) biphenyl and t-butyloxycarboryl-L-proline as initial raw materials, the initial raw materials are separately synthesized into a midbody N-4, N-3, N-2 and N-1 in four steps, and finally a product daclatasvir is produced. The synthetic method of the invention has the advantages of short production process, high product yield, low cost, easiness in acquiring raw materials and suitability for industrialized mass production.
Owner:ANHUI YELLEN PHARMA
A kind of method for preparing daclatasvir
The invention provides a method for preparing Daclatasvir. According to the method disclosed by the invention, N-(methoxycarbonyl)-L-valine and 5,5'-(4,4'-biphenylyl)di(2-((2S)-2- pyrrolidinyl)-1hydrogen-imidazole)tetrahydrochloride are used as reaction materials, under the participation of an activating agent namely 1-(3-dimethylaminopropyl)-3-ethyl carbodiimide hydrochloride and an alkaline reagent, ethyl cyanoglyoxylate-2-oxime is used as a reaction catalyst, and a reaction is performed in an alcohol solvent. The method for preparing the Daclatasvir provided by the invention is simple and convenient to operate, economic and cheap, environmental-friendly, safe, high in recovery rate and suitable for industrialized production.
Owner:SUNSHINE LAKE PHARM CO LTD
A kind of refining method of daclatasvir hydrochloride
ActiveCN109305962BEasy to operateReduce manufacturing costOrganic chemistryActivated carbonPhysical chemistry
The invention discloses a method for refining daclatasvir hydrochloride, which comprises the following steps: (1) adding crude daclatasvir hydrochloride to methanol and dissolving at 55-65°C; (2) adding activated carbon at 55-65°C (3) Filtration, the resulting filtrate is warmed up to 55-65°C, and the mixed solvent of water-soluble aprotic solvent A and protic solvent B is added dropwise for crystallization. ) filtering step (3) stirring the crystallized mixture, and vacuum-drying the obtained filter cake to obtain high-purity daclatasvir hydrochloride. The method of the present invention can effectively remove the impurities in the crude product of daclatasvir hydrochloride, the product purity is above 99.5%, the isomer impurities and other single impurities are not more than 0.1%, and the refining yield is not less than 85%. In addition, the present invention The refining method has a simple and convenient process, and the process is easy for industrialized production.
Owner:YANGTZE RIVER PHARM GRP CO LTD
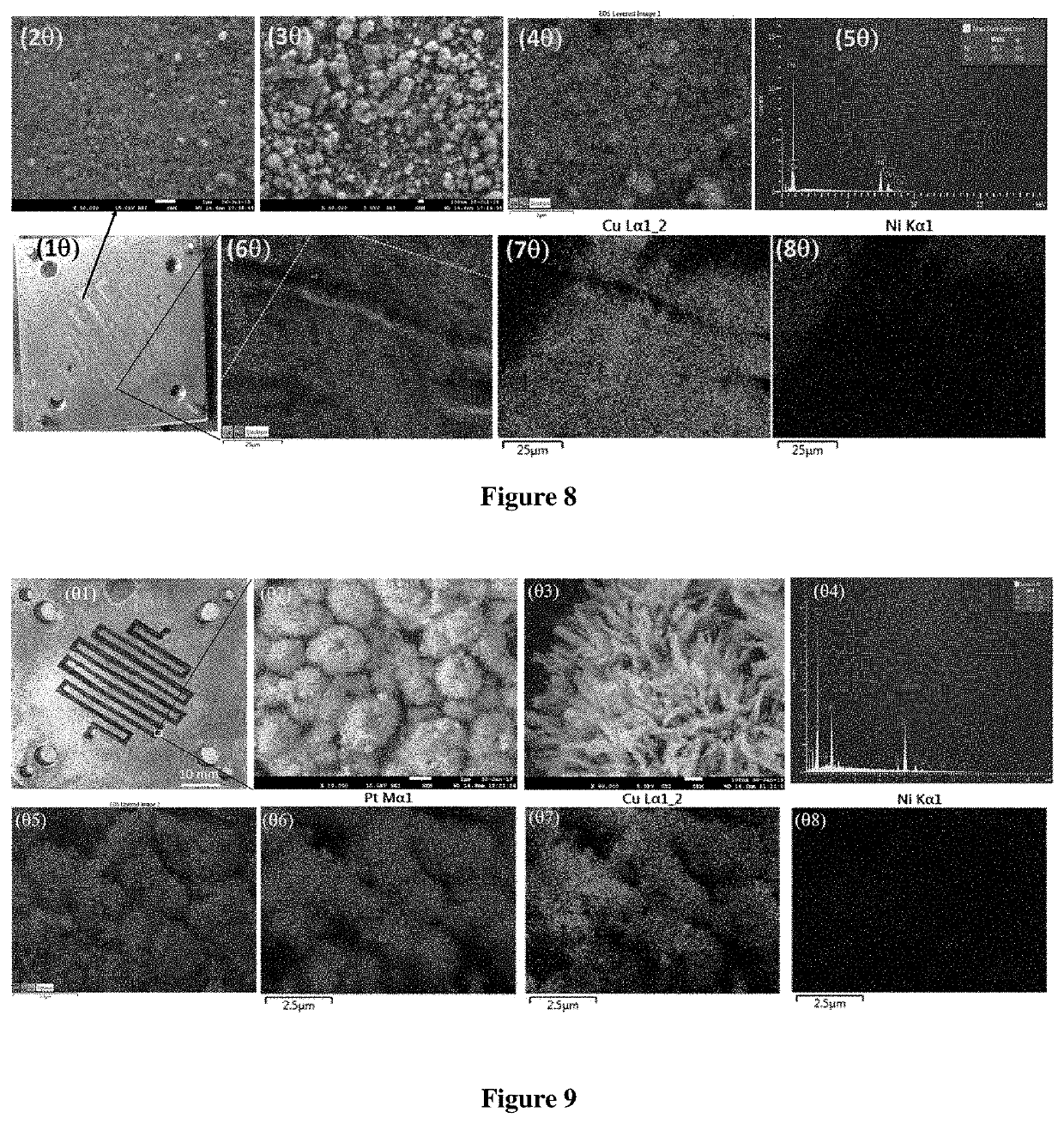
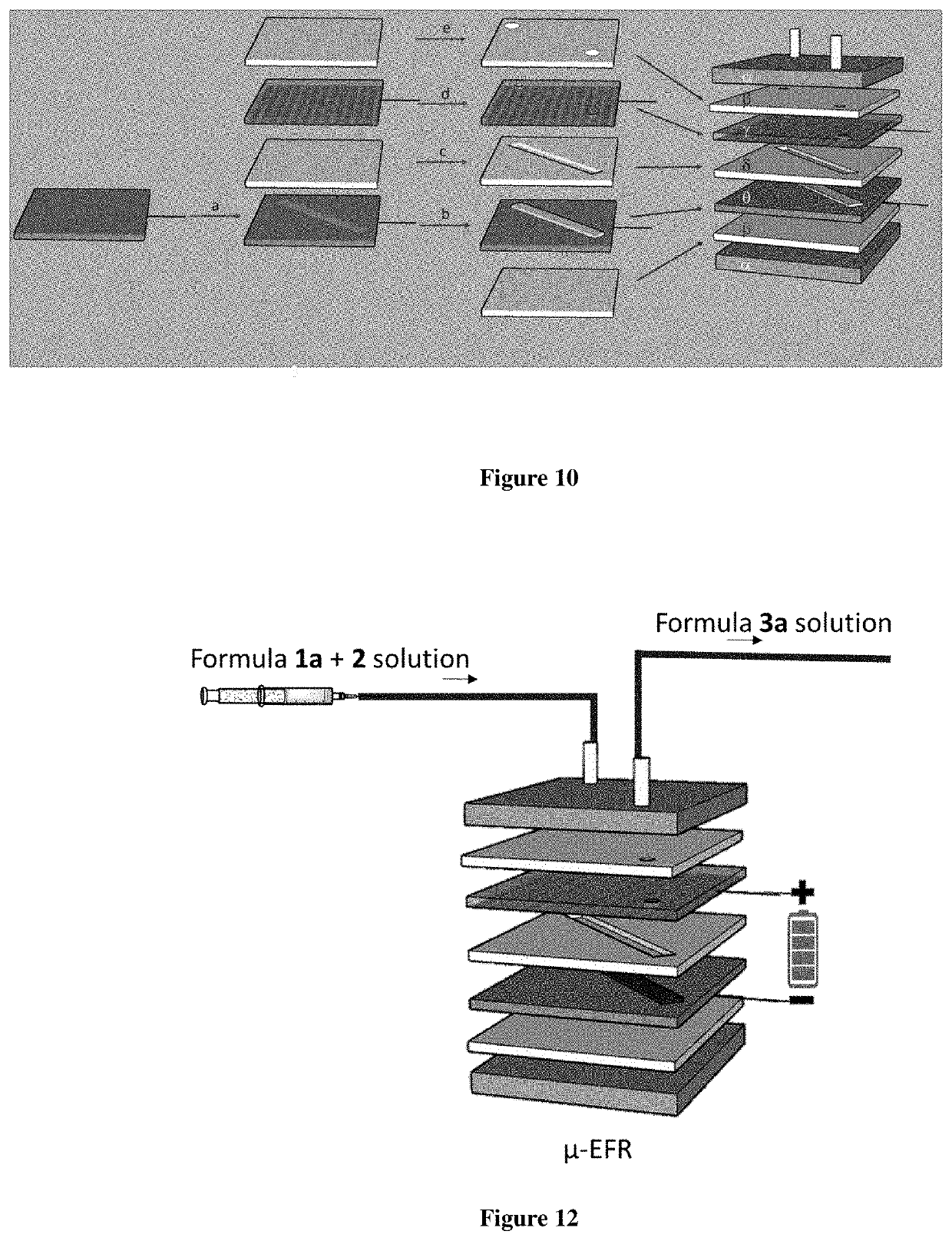
Micro-electrolysis reactor for ultra fast, oxidant free, C—C coupling reaction and synthesis of daclatasvir analogs thereof
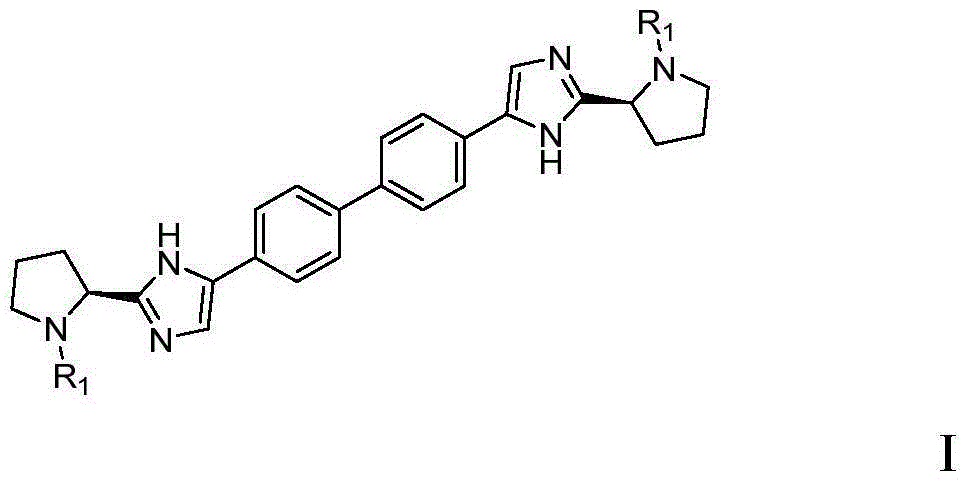
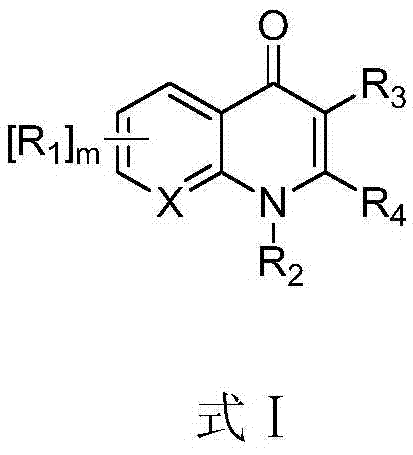
The present invention relates to a continuous micro-electro-flow reactor system for ultra-fast, oxidant free, C—C coupling reaction for making symmetrical biaryls and analogs thereof. This invention further relates to the said process for preparation of antiviral drug, daclatasvir of general formula I.
Owner:COUNCIL OF SCI & IND RES
Method for preparing compound
InactiveCN107663196AIncreased efficiency of coupling reactionsLow costOrganic chemistryPhosphateTriflic acid
The invention provides a method for preparing a compound shown in formula I. The method comprises the steps as follows: a compound shown in formula II is contacted with a compound shown in formula III, so that the compound shown in formula I is obtained, wherein X1 is Cl, Br, I, trifluoromethanesulfonic acid and enol phosphate; R is alkyl, hydroxyl or alkoxy. The compound shown in the formula is an intermediate DSV103 of daclatasvir. The method for preparing the intermediate DSV103 has the characteristics of being high in purity, high in yield, low in cost, simple to operate, small in toxicityand pollution and stable in process, the yield can reach 60%-80%, and the purity can reach 95% or above. Daclatasvir can be further obtained from the daclatasvir intermediate obtained with the preparation process, and the obtained daclatasvir has higher purity and yield than the prior art.
Owner:SUNSHINE LAKE PHARM CO LTD
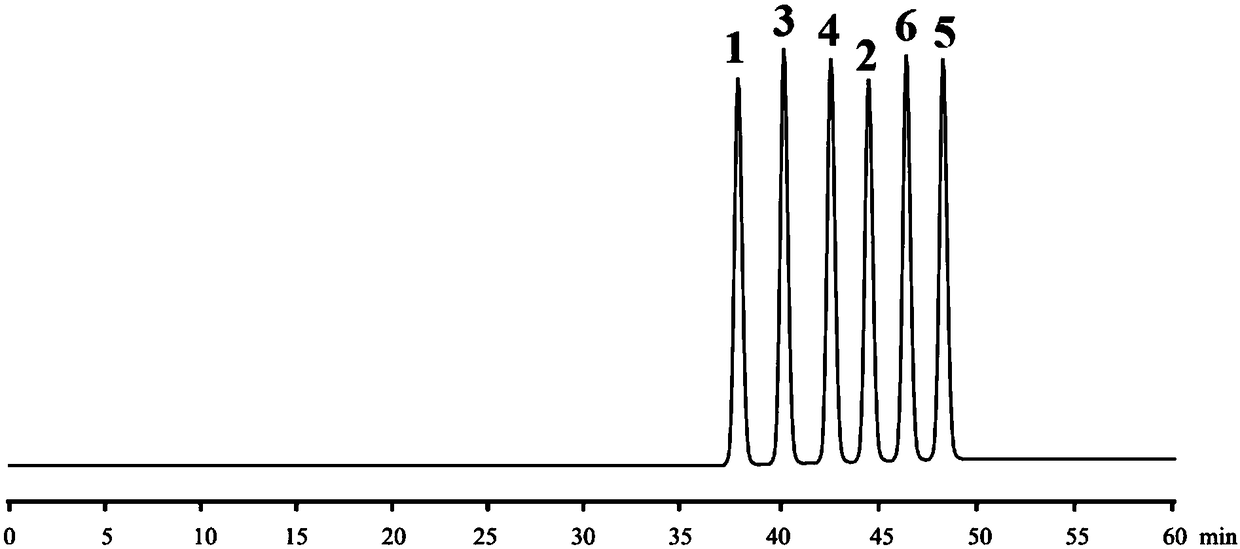
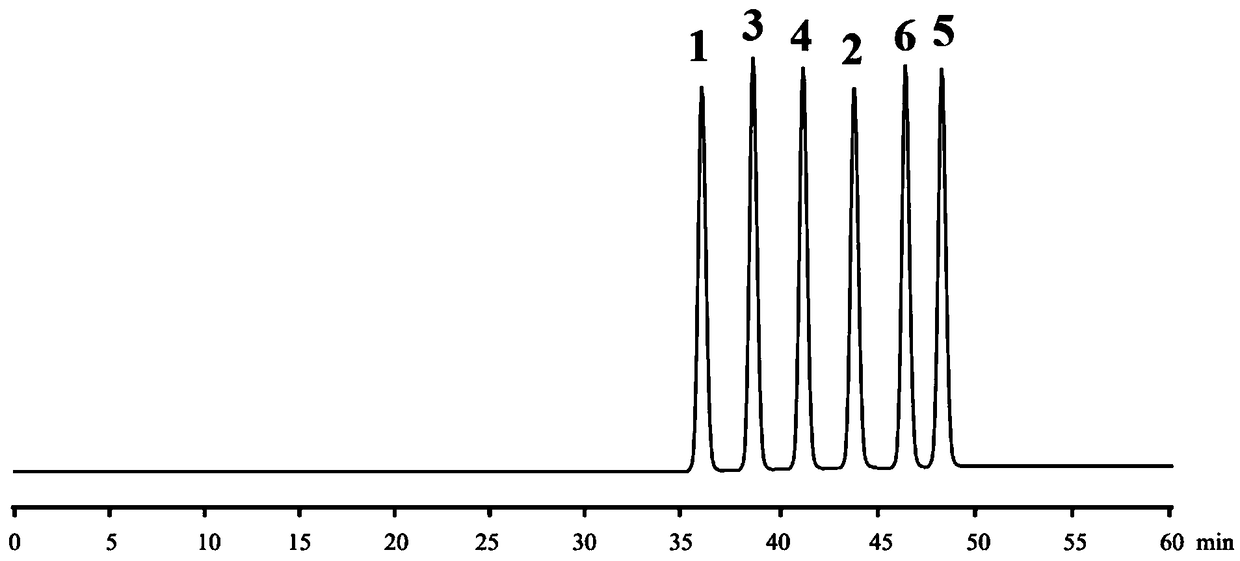
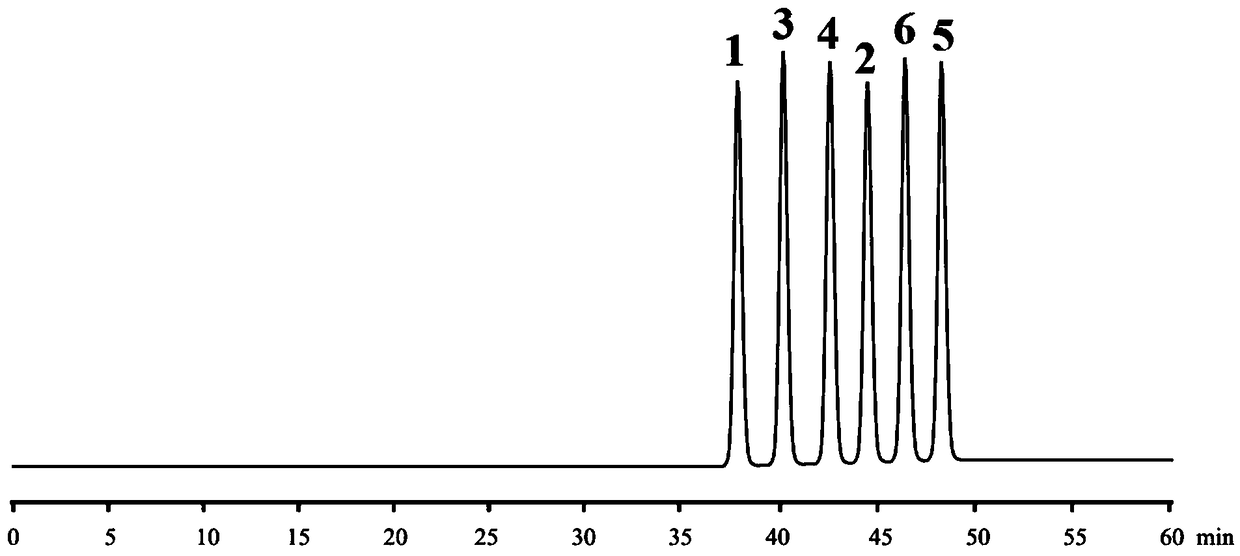
High performance liquid chromatography and chiral mobile phase separated hydrochloric acid daclatasvir and five optical isomers thereof
The invention discloses a high performance liquid chromatography and chiral mobile phase separated hydrochloric acid daclatasvir and five optical isomers thereof. The high performance liquid chromatography and chiral mobile phase separated hydrochloric acid daclatasvir is characterized in that L-asparaginic acid, (2R, 3R)-dipropyl tartaric acid, D-(+)-Di-p-toluoyl-L-tartaric acid, (1R, 2R)-(-)-N-(p-methylphenylsulfonyl)-1, 2-diphenylethanediamine or L-valyl-L-tyrosine is used as an additive and added to the mobile phase, and a common C18 liquid chromatographic column can be utilized to effectively separate hydrochloric acid daclatasvir and five optical isomers thereof, and mutual baseline separation is achieved. The technicians in the field know that the chiral mobile phase is limited andcan only be used for separating limited compounds, and systemic theory guidance is not provided to guide any compound to be separated through the chiral mobile phase, and the chiral mobile phase for separating is only performed by 'trial and error' repeated testing and random exploring. Therefore, the method is not obvious to the technicians in the field and has the creativity as specified by thepatent law.
Owner:覃叶枫
Amorphous form of daclatasvir and its salts and process for preparation thereof
This relates to an amorphous form of daclatasvir and its salts and process for preparation thereof. In particular, it relates to an amorphous form of daclatasvir and daclatasvir dihydrochloride. It also relates to pharmaceutical compositions comprising an amorphous form of daclatasvir or daclatasvir dihydrochloride for oral administration for treatment of Hepatitis C virus (HCV).
Owner:CADILA HEALTHCARE LTD
Method for preparing Daclatasvir
The invention provides a method for preparing Daclatasvir. According to the method disclosed by the invention, N-(methoxycarbonyl)-L-valine and 5,5'-(4,4'-biphenylyl)di(2-((2S)-2- pyrrolidinyl)-1hydrogen-imidazole)tetrahydrochloride are used as reaction materials, under the participation of an activating agent namely 1-(3-dimethylaminopropyl)-3-ethyl carbodiimide hydrochloride and an alkaline reagent, ethyl cyanoglyoxylate-2-oxime is used as a reaction catalyst, and a reaction is performed in an alcohol solvent. The method for preparing the Daclatasvir provided by the invention is simple and convenient to operate, economic and cheap, environmental-friendly, safe, high in recovery rate and suitable for industrialized production.
Owner:SUNSHINE LAKE PHARM CO LTD
A kind of method for separating and detecting daclatasvir hydrochloride and its optical isomers
ActiveCN108732280BSimple and effective separation assayComponent separationPotassium hexafluorophosphateCellulose
The invention specifically relates to a method for separating and detecting daclatasvir hydrochloride and its optical isomers. A method for separating and measuring daclatasvir hydrochloride and its optical isomers (impurities) by liquid chromatography, characterized in that, using cellulose tris(3,5-dimethylphenylcarbamate) It is a chiral chromatographic column with fillers, and the mobile phase is a mixed solution of sodium hexafluorophosphate, potassium hexafluorophosphate, formic acid, acetic acid, phosphoric acid or phosphate aqueous solution and an organic phase of acetonitrile or methanol. The separation and detection method of the present invention can effectively separate daclatasvir hydrochloride and its optical isomers (impurities), the separation degree reaches more than 3.0, and complete baseline separation, so that the quality of daclatasvir hydrochloride can be accurately and effectively controlled. By adopting the separation method of the present invention, the time for separating and detecting daclatasvir hydrochloride and its optical isomers is within 30-80 minutes, and the method of the present invention has the advantages of simplicity, rapidity, accuracy and the like.
Owner:SUNSHINE LAKE PHARM CO LTD
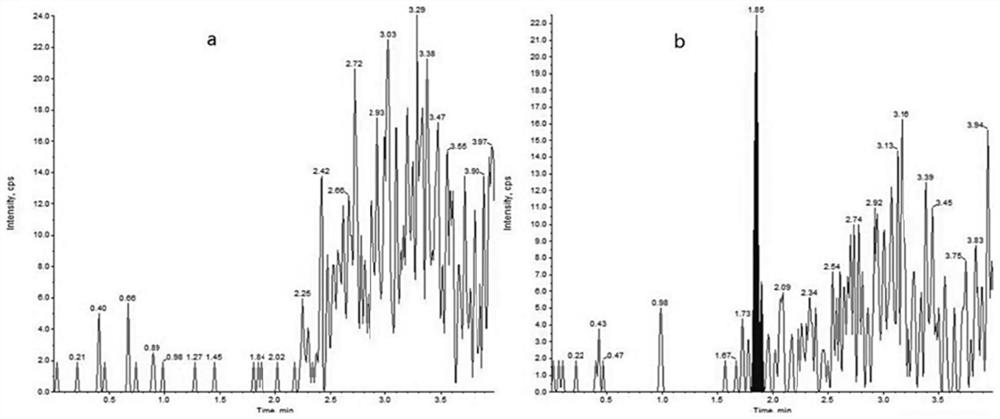
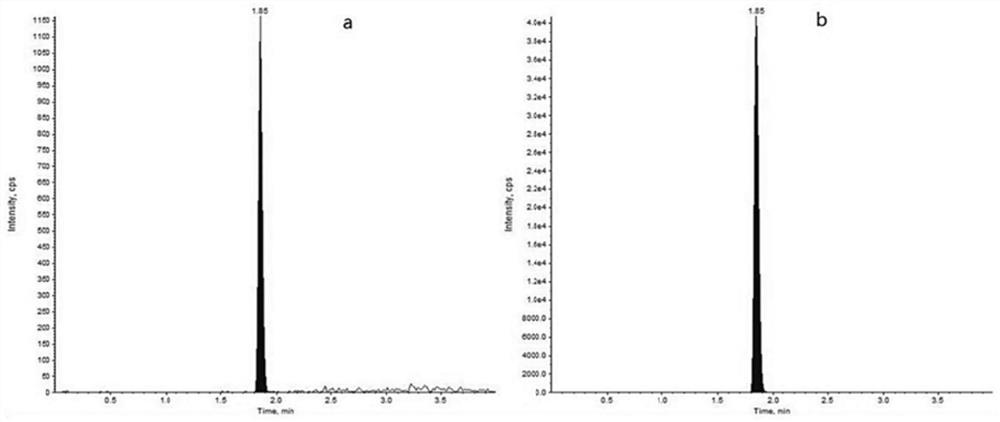
Quantitative detection method of daclatasvir in beagle plasma
PendingCN112285229AImprove qualitative accuracyHigh sensitivityComponent separationQuantitative determinationInternal standard
The invention discloses a quantitative detection method of daclatasvir in beagle plasma, and belongs to the technical field of drug detection. The quantitative detection method comprises the followingsteps of: 1) preparing a daclatasvir series working solution and a daclatasvir-d6 internal standard working solution; 2) pretreating to-be-detected beagle plasma; 3) preparing a series of calibrationstandard samples and accompanying quality control samples; and (4) measuring the concentration of the daclatasvir in the to-be-measured beagle plasma, wherein a standard curve is drawn by taking a chromatographic peak area ratio of the daclatasvir to the internal standard daclatasvir-d6 as a vertical coordinate and taking the concentration of the daclatasvir in the beagle blank plasma as a horizontal coordinate, and the concentration of the daclatasvir in the to-be-measured beagle plasma is calculated according to the standard curve. According to the quantitative detection method, a protein precipitation method is adopted to treat a beagle plasma sample, an electrospray ion source is used as an ionization technology, the detection method is simple and convenient to operate, high in sensitivity, good in reproducibility, high in selectivity, low in detection limit, short in analysis time and suitable for high-throughput quantitative determination of the beagle plasma sample and preclinical beagle in-vivo pharmacokinetic research.
Owner:TONGJI HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI TECH
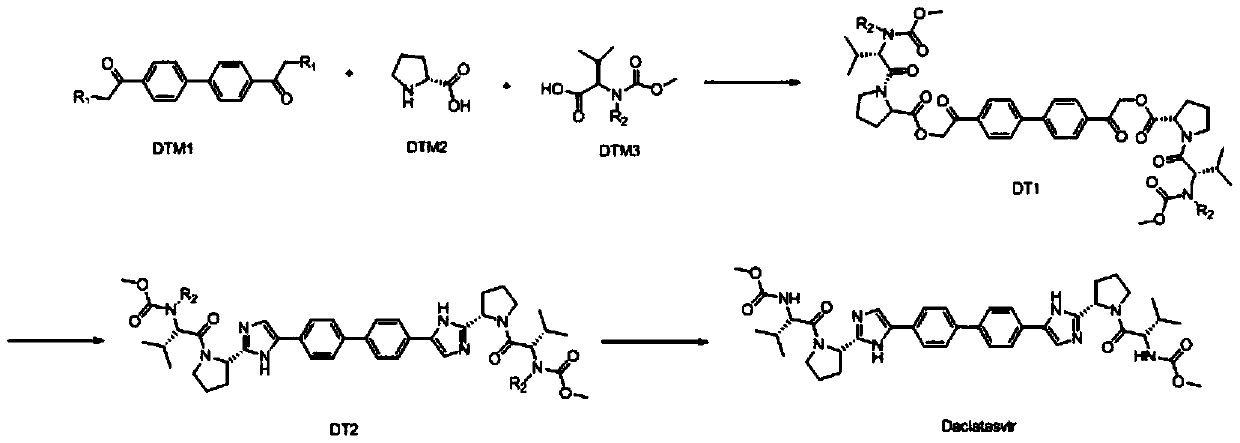
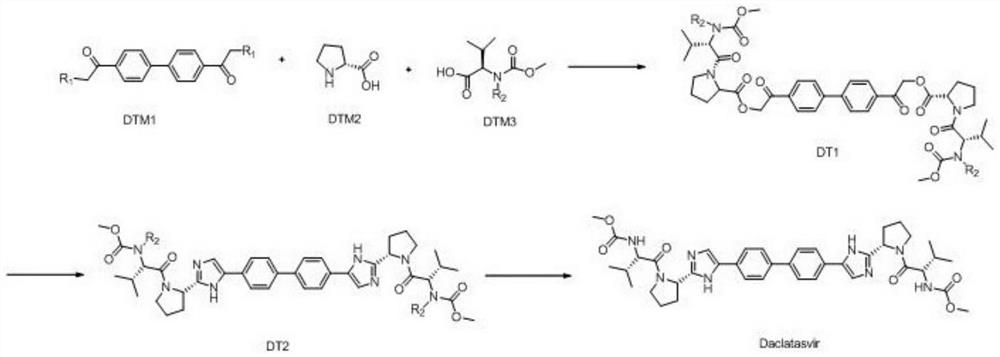
One-pot preparation technology of NS5A protein inhibitor daclatasvir
ActiveCN110878090AHigh yieldSimple processOrganic chemistry methodsBulk chemical productionBiochemical engineeringChemical compound
The invention discloses a one-pot preparation technology of an NS5A protein inhibitor daclatasvir. The technology mainly comprises the following three steps: 1) preparing an intermediate DT1: carryingout a butt reaction on reaction initial raw materials which are DTM1, DTM2 and DTM3 under the action of an alkali reagent and a condensing agent to prepare the intermediate DT1; 2) preparing an intermediate DT2: cyclizing the intermediate DT1 and an amine compound to prepare the intermediate DT2; and 3) preparing daclatasvir: removing an amino protecting group from the intermediate DT2 by using an amino protecting group removing reagent to prepare the product daclatasvir. The method for preparing daclatasvir has the characteristics of high product yield, simple and easy-to-operate overall process, no strong corrosive substances in the preparation materials, effectiveness in safety ensuring, and suitableness for large-scale industrial production.
Owner:NANTONG CHANGYOO PHARMATECH CO LTD
A compound and its application in the preparation of anti-hepatitis C virus medicine
ActiveCN106946775BRich diversityEasy to separate and purifyOrganic chemistryAntiviralsPharmaceutical SubstancesBiology
The present invention discloses a compound and applications of the compound in preparation of anti-hepatitis C virus drugs, wherein the structure formula of the compound is represented by a formula I or II, and the compound represented by the formula I or compound represented by the formula II can be subjected to drug combination with other anti-virus drugs such as interferon (PEG IFN-[alpha]), ribavirin (RBV), boceprevir, telaprevir, simeprevir, sofosbuvir, daclatasvir and the like t prepare anti-HCV products and other anti-virus infection products. According to the present invention, the compound has rich functional group diversity and modificability, and the product is relatively easy to separate and purify; the compounds can well inhibit HCV and other viruses, are obtained through phenotype screening, have different antiviral mechanisms, have extremely novel and innovated structures in the anti-virus field, and are not reported in the prior art; and the compound of the present invention has broad development and application prospect.
Owner:TSINGHUA UNIV
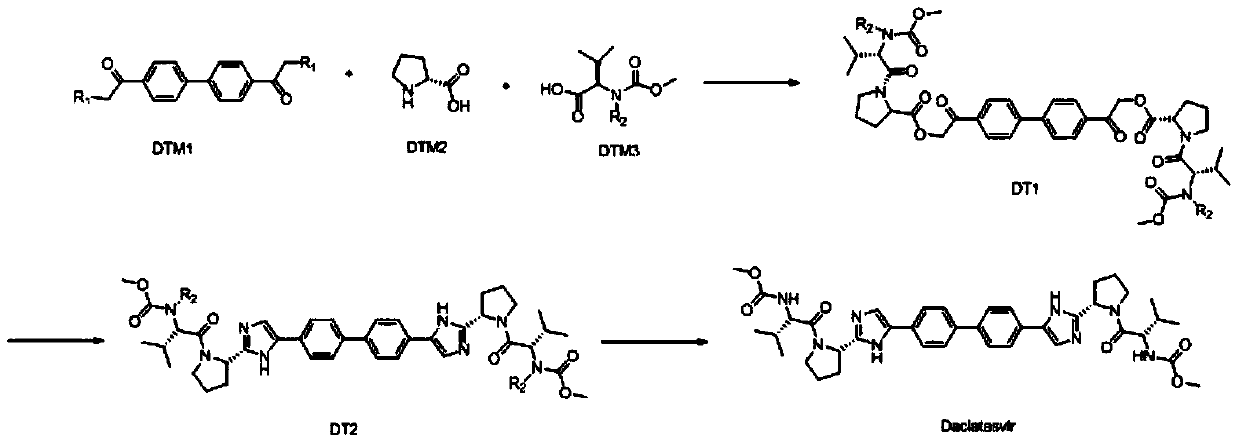
One-pot preparation process of ns5a protein inhibitor-daclatasvir
ActiveCN110878090BHigh yieldSimple processOrganic chemistry methodsBulk chemical productionBiochemical engineeringChemical compound
The invention discloses a one-pot preparation process of NS5A protein inhibitor daclatasvir, which mainly includes three steps, 1) preparation of intermediate DT1: the reaction starting materials DTM1, DTM2 and DTM3 are mixed with an alkali reagent and a condensation agent 2) Preparation of intermediate DT2: cyclization of intermediate DT1 with an amine compound to obtain intermediate DT2; 3) Preparation of Daclatasvir: Intermediate DT2 was deaminated with a protecting group reagent The product Daclatasvir is prepared by removing the amino protecting group. Using the method to prepare daclatasvir has the characteristics of high product yield, simple and easy operation of the overall process, and no strong corrosive substances in the preparation material, the safety is effectively guaranteed, and it is suitable for large-scale industrial production.
Owner:NANTONG CHANGYOO PHARMATECH CO LTD
Micro-electrolysis reactor for ultra fast, oxidant free, c-c coupling reaction and synthesis of daclatasvir analogs thereof
The present invention relates to a continuous micro-electro-flow reactor system for ultra-fast, oxidant free, C—C coupling reaction for making symmetrical biaryls and analogs thereof. This invention further relates to the said process for preparation of antiviral drug, daclatasvir of general formula I.
Owner:COUNCIL OF SCI & IND RES
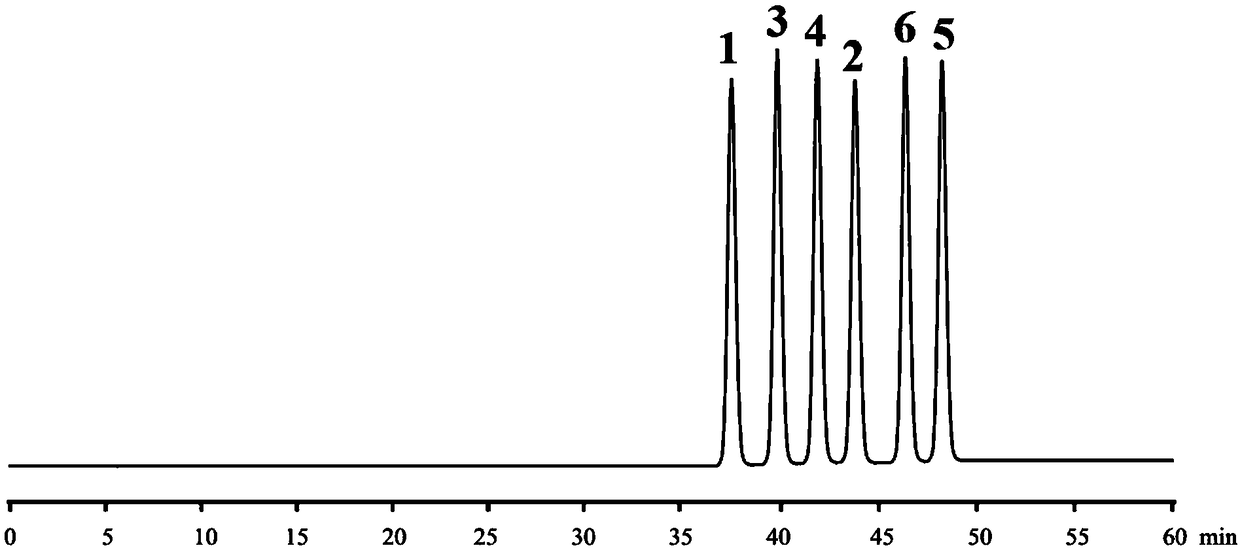
High performance liquid chromatography chiral mobile phase method for separation of daclatasvir hydrochloride and five optical isomers thereof
InactiveCN108956799ALow costImprove applicabilityComponent separationSolid sorbent liquid separationTyrosineTrial and error
The invention discloses a high performance liquid chromatography chiral mobile phase method for separation of daclatasvir hydrochloride and five optical isomers thereof. The daclatasvir hydrochlorideand the five optical isomers thereof can be effectively separated by adding of L-aspartic acid, (2R, 3R) bis-n-propyl tartaric acid, (+)-di-p-toluoyl-D-tartaric acid, and (1R, 2R)-(-)-N-(p-toluenesulfonyl)-1,2-diphenylethylenediamine or L-valyl-L-tyrosine as additives to a mobile phase by use of a common C18 liquid chromatography column, and baseline resolution of every two of the daclatasvir hydrochloride and the five optical isomers thereof can be achieved. Persons skilled in the art know that a chiral mobile phase method is very limited in that and only a limited number of compounds can beseparated, no systematic theory can guide which compounds can be separated by the chiral mobile phase method, and only a trial-and-error method can be used for repeatedly trying and groping without rules. Accordingly, the high performance liquid chromatography chiral mobile phase method has obvious creativity prescribed by the patent law for the persons skilled in the art.
Owner:覃叶枫
Preparation method for daclatasvir
The invention discloses a preparation method for daclatasvir. The preparation method comprises the following steps: in a solvent, performing a cyclization reaction on a compound shown as a formula D10 and ammonium acetate, so as to prepare the daclatasvir shown as a formula I, wherein the cyclization reaction is shown in the description. The preparation method for daclatasvir is low in cost, high in yield, friendly to environment, simple in steps, mild in reaction conditions and suitable for industrialized production.
Owner:SHANGHAI INST OF PHARMA IND +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com