Method for preparing Daclatasvir
A compound and alcohol solvent technology, which is applied in the synthesis field of daclatasvir, can solve the problems of production personnel's personal health threat, non-recyclable, high toxicity of acetonitrile, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
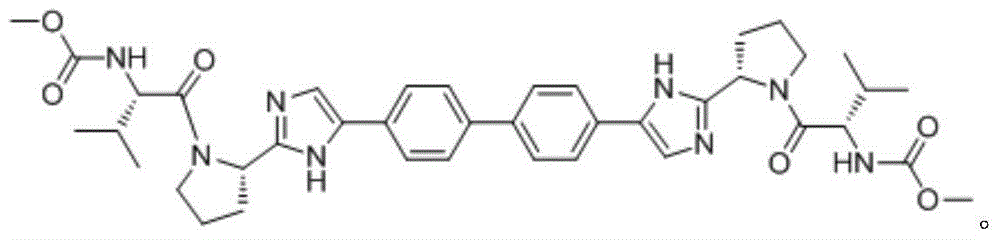
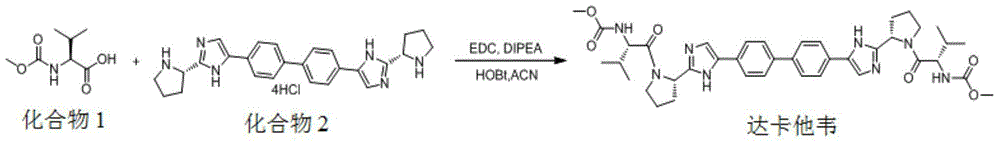
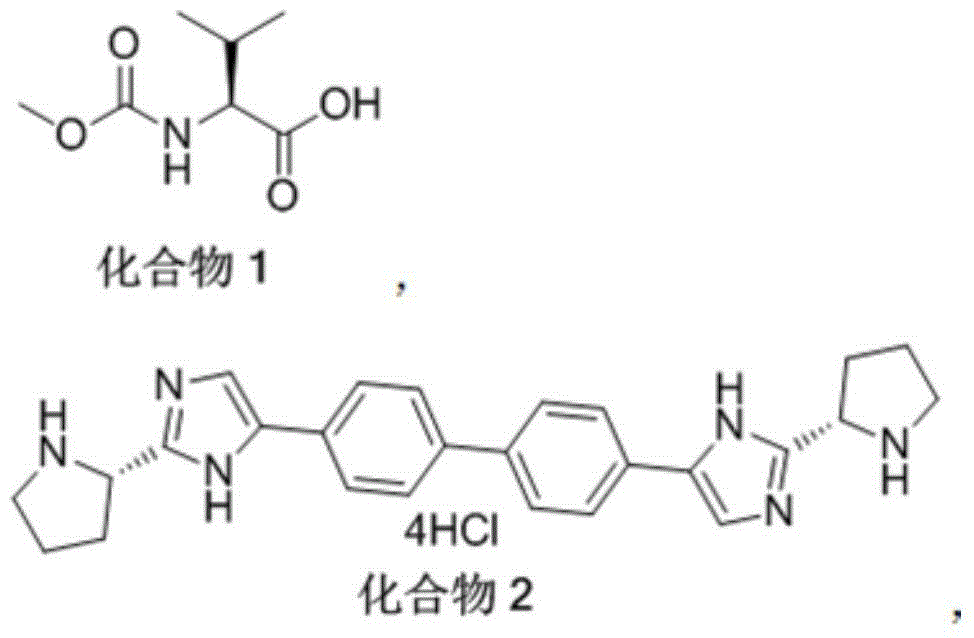
[0021] The method for preparing daclatasvir compound provided by the present invention, the method uses compound 1 and compound 2 as reaction materials,
[0022]
[0023] With the participation of activators and alkaline reagents, ECA was used as a reaction catalyst, and the synthesis reaction was carried out in alcoholic solvents.
[0024] In some embodiments, the alcoholic solvent may be methanol, ethanol, isopropanol, propanol, butanol, isobutanol or ethanol-water mixture.
[0025] In other embodiments, the activator may be EDC.
[0026] In other embodiments, the alkaline reagent can be DIPEA, triethylamine, N-methylmorpholine, sodium hydroxide, potassium carbonate, sodium carbonate or potassium hydroxide.
[0027] In some embodiments, first add alcohol solvent to the reaction vessel, then control the temperature of the reaction solvent at -10°C to 35°C, add compound 1, compound 2, ECA, activator and alkaline reagent; The temperature of the reaction system is 15°C-45°C...
specific Embodiment approach
[0037] In order to enable those skilled in the art to better understand the technical solutions of the present invention, some non-limiting examples are further disclosed below to further describe the present invention in detail.
[0038] The reagents used in the present invention can be purchased from the market or can be prepared by the methods described in the present invention.
Embodiment 1
[0040]
[0041] Add 450mL ethanol and DIPEA (142.8g, 1.105mol) to the reaction flask at room temperature, and add compound 2 (150.0g, 0.263mol), compound 1 (106.0g, 0.605mol), ECA (15.0 g, 0.106 mol), EDC (116.0 g, 0.605 mol). After adding the materials, control the temperature at 35°C and react for 2h to 4h. Use thin-layer chromatography to detect the complete reaction of the raw materials. Add the reaction liquid dropwise to 2250mL water. After the dropwise addition, control the temperature at 10°C to 30°C and stir for 1h, then filter to obtain daclatasvir , 212.6g was obtained after drying, and the yield was 99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com