Novel method for synthesizing daclatasvir intermediate
A cyclization and compound technology, which is applied in the field of preparation of daclatasvir intermediates, can solve the problems of rising raw material costs, many steps, and poor safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0064] Preparation of daclatasvir intermediate
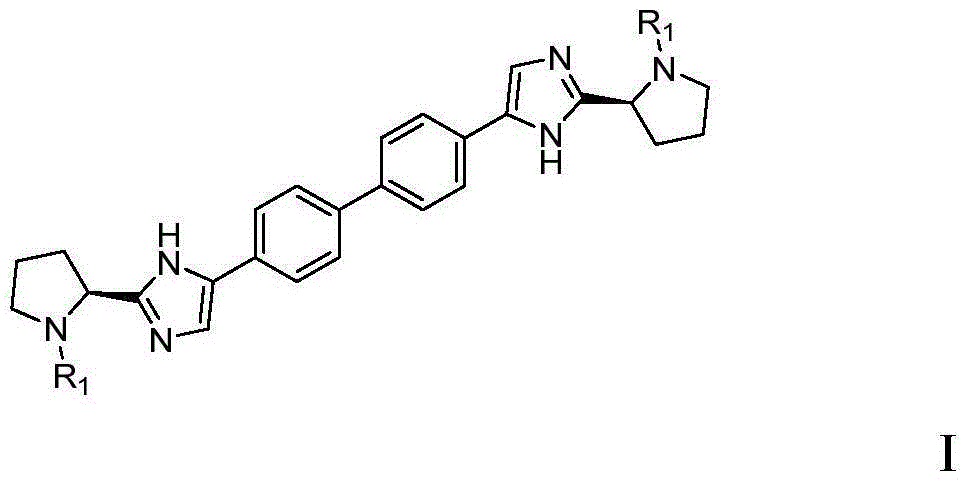
[0065] The present invention provides a daclatasvir intermediate, the preparation method of the compound shown in formula 1,
[0066]
[0067] Described method comprises the following steps:
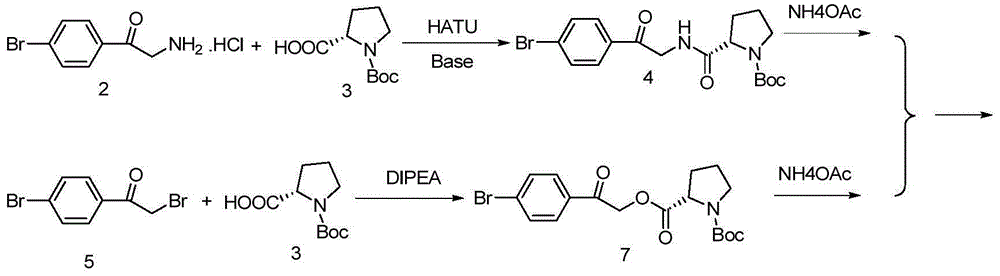
[0068] i) under the action of a catalyst, the compound of formula II reacts with the compound of formula III to obtain the compound of formula IV;
[0069]
[0070] and
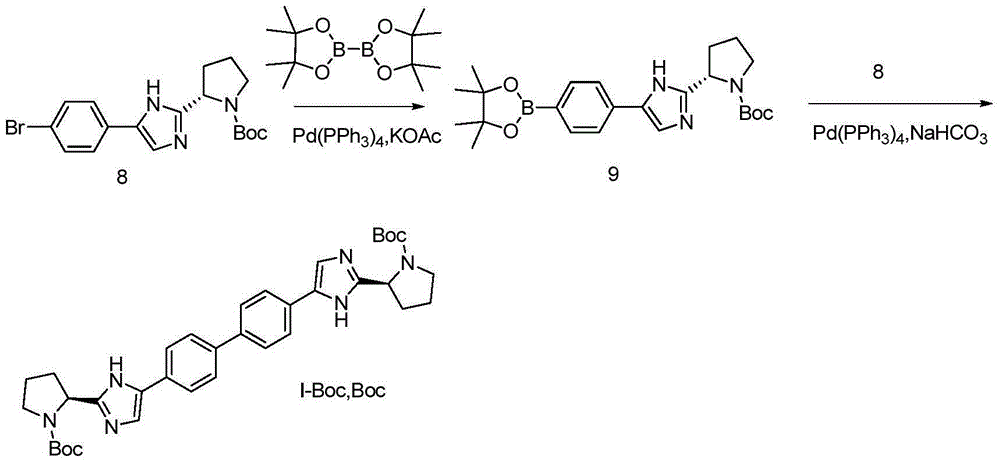
[0071] The compound of formula I is prepared from the compound of formula IV; wherein, the preparation can be prepared by referring to existing methods in the art, or designed by those skilled in the art based on common knowledge in the art.
[0072] x 1 、X 2 Each is independently selected from the following group: Cl, Br, I.
[0073] More preferably, the method comprises the following steps:
[0074] i) under the action of a catalyst, the compound of formula II reacts with the compound of formula III to obtain the compound of formula IV;
[0075]
[0076] i...
Embodiment 1
[0134]
[0135] Add 21.7g (163.0mmol, 2.5eq) of aluminum trichloride and 80ml of dichloromethane into a 250ml four-neck flask, start stirring, and slowly add 10.0g of biphenyl (65.0mmol, 1.0eq) dropwise at 20-25°C and A mixed solution consisting of 20ml of dichloromethane was stirred for 30 minutes until most of the aluminum chloride was dissolved.
[0136] The reaction mixture was cooled to 0°C, and 22.0g (194.8mmol, 3.0eq) of chloroacetyl chloride was slowly added dropwise. After the dropwise addition was completed, it was kept at 0°C for 1 hour, then naturally warmed to room temperature, and continued to stir for 2 hours. TLC tracking showed that the raw material disappear.
[0137] The reaction solution was poured into 100ml of 10% ice water-hydrochloric acid mixture, stirred for 30 minutes, and the organic layer was separated. The organic phase was washed with water, 10% sodium bicarbonate solution, saturated brine, dried over anhydrous sodium sulfate, and concentrate...
Embodiment 2
[0140]
[0141] Put 30.0g (97.7mmol, 1.0eq) of IV-Cl, Cl and 62.2g (107.5mmol, 2.2eq) of compound 11 in 500ml of acetone, start stirring, heat to 50-55°C for 2h, TLC shows that the raw materials disappear, concentrate To dryness, add 300ml of water, extract 3 times with 200mlX3 toluene, combine the toluene layers, wash with 300ml of water, dry over anhydrous sodium sulfate, filter, and the obtained toluene solution is directly used in the next reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com