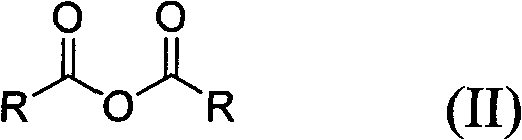Method for preparing symmetrical acid anhydride
A symmetrical, acid anhydride technology, applied in the preparation of carboxylic acid anhydrides, organic chemical methods, chemical instruments and methods, etc., can solve the problems of unstable catalysts, low reaction yields, severe conditions, etc., and achieve good promotion and application prospects and advanced process routes. , the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: the preparation of propionic anhydride
[0024] The amount ratio of the feed material is carboxylic acid RCOOH: bis(trichloromethyl)carbonate: the catalyst is 1:0.17:0.01, RCOOH is propionic acid, the catalyst is N,N-dimethylformamide, and the organic solvent is ethyl acetate , its dosage is 3 times of the mass of carboxylic acid.
[0025] In a 250mL three-necked flask equipped with a thermometer, a reflux condenser and mechanical stirring, add 7.4g (100mmol) of propionic acid, 5.0g (17mmol) of bis(trichloromethyl)carbonate, 22.2ml of ethyl acetate and N,N - Dimethylformamide 0.07 g (1 mmol). After the addition was completed, the temperature was raised to 60-65° C., and the reaction was kept for 6 hours. After the reaction was completed, the solvent was evaporated under reduced pressure, and then 6.3 g of propionic anhydride was obtained by high vacuum distillation. The product yield was 97.0%, and it was an oily substance. 1 H-NMR (400MHz, CDCl 3 ): 1....
Embodiment 2
[0026] Embodiment 2: the preparation of benzoic anhydride
[0027] The amount ratio of feed material carboxylic acid RCOOH: two (trichloromethyl) carbonate: catalyst is 1:0.17:0.005, and RCOOH is benzoic acid, and catalyst is N, N-dimethylformamide, and organic solvent is toluene, and The dosage is twice the mass of carboxylic acid.
[0028] In a 250mL three-necked flask equipped with a thermometer, a reflux condenser and a mechanical stirrer, add 12.2g (100mmol) of benzoic acid, 17g (17mmol) of bis(trichloromethyl)carbonate, 24.4ml of toluene and N,N-dimethyl 0.04 g (0.5 mmol) of methyl formamide. After the addition was completed, the temperature was raised to 70-75°C, and the temperature was kept for 5 hours. After the reaction, the solvent was evaporated under reduced pressure, and the residue was recrystallized with cyclohexane to obtain 11.0 g of benzoic anhydride. The product yield was 97.0%, and it was a solid. Melting point: 43-44°C. 1 H-NMR (400MHz, CDCl 3 ): 8.12...
Embodiment 3
[0029] Embodiment 3: the preparation of o-chlorobenzoic anhydride
[0030] Replace benzoic acid with o-chlorobenzoic acid, the reaction solvent is dichloromethane, the reaction temperature is 40~45°C, and the reaction time is 10h. The operation process is the same as in Example 2 to obtain o-chlorobenzoic anhydride with a yield of 94%, solid . Melting point: 79-81°C. 1 H-NMR (400MHz, CDCl 3 ): 8.04 (dd, 2H, J=2, 16Hz), 7.54-7.48 (4H, m), 7.42-7.26 (2H, m); IR (KBr, cm -1 ): 1780, 1718; MS(EI): m / z(%)=259(M+1, 56), 141(39), 139(100), 113(17), 111(44), 75(18 ). 13 C NMR (100MHz, CDCl 3 ): 160.34, 135.1, 134.2, 132.6, 131.6, 127.8, 126.9.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com