Method for preparing daclatasvir
A technology for finished products and compounds of viril, applied in the field of preparation of daclatasvir, which can solve the problem of low yield of daclatasvir
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
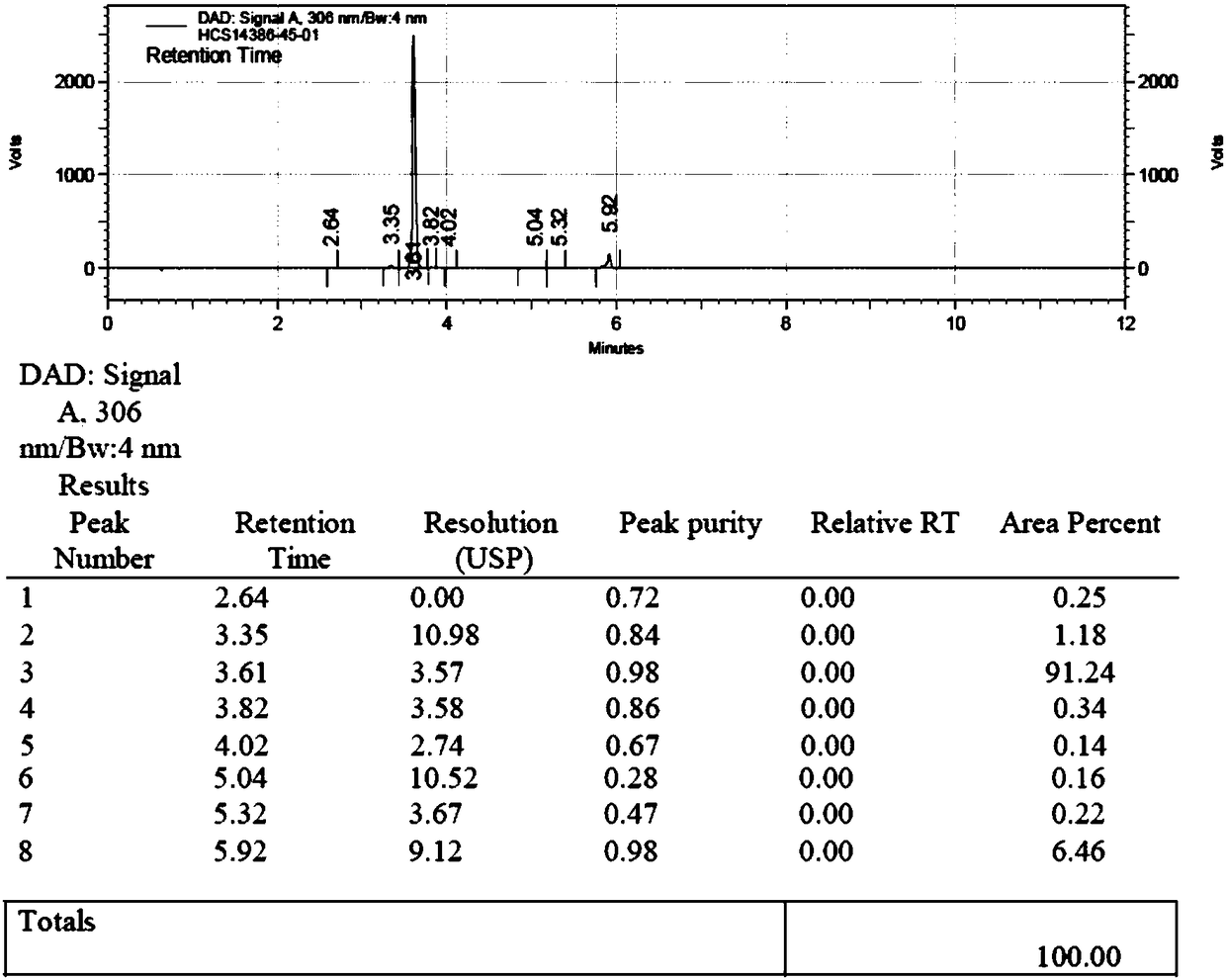
[0047] Feed intake 5g of the compound shown in formula II, 3.5g of the compound shown in formula III, add 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride of 2.5 times the molar amount of the compound shown in formula II Salt and dichloromethane 20ml, HPLC detection reaction. After the reaction (the compound shown in formula II≤0.5%), add 10ml of ammonia water, react at 30°C for 1h, and detect the reaction by thin-layer chromatography (developing solvent is dichloromethane:methanol=8:1), and wait for the reaction shown in formula 1 After the compound and the compound shown in formula 2 are completely converted (the compound shown in formula 1 and the compound shown in formula 2 are both ≤0.5%) into daclatasvir, add 30ml of dichloromethane and 25ml of water, extract and separate, and separate to obtain the organic phase After the organic layer was concentrated to dryness, the finished product of daclatasvir was obtained: 6.38g, the yield was 98.5%, and the HPLC purity...
Embodiment 2
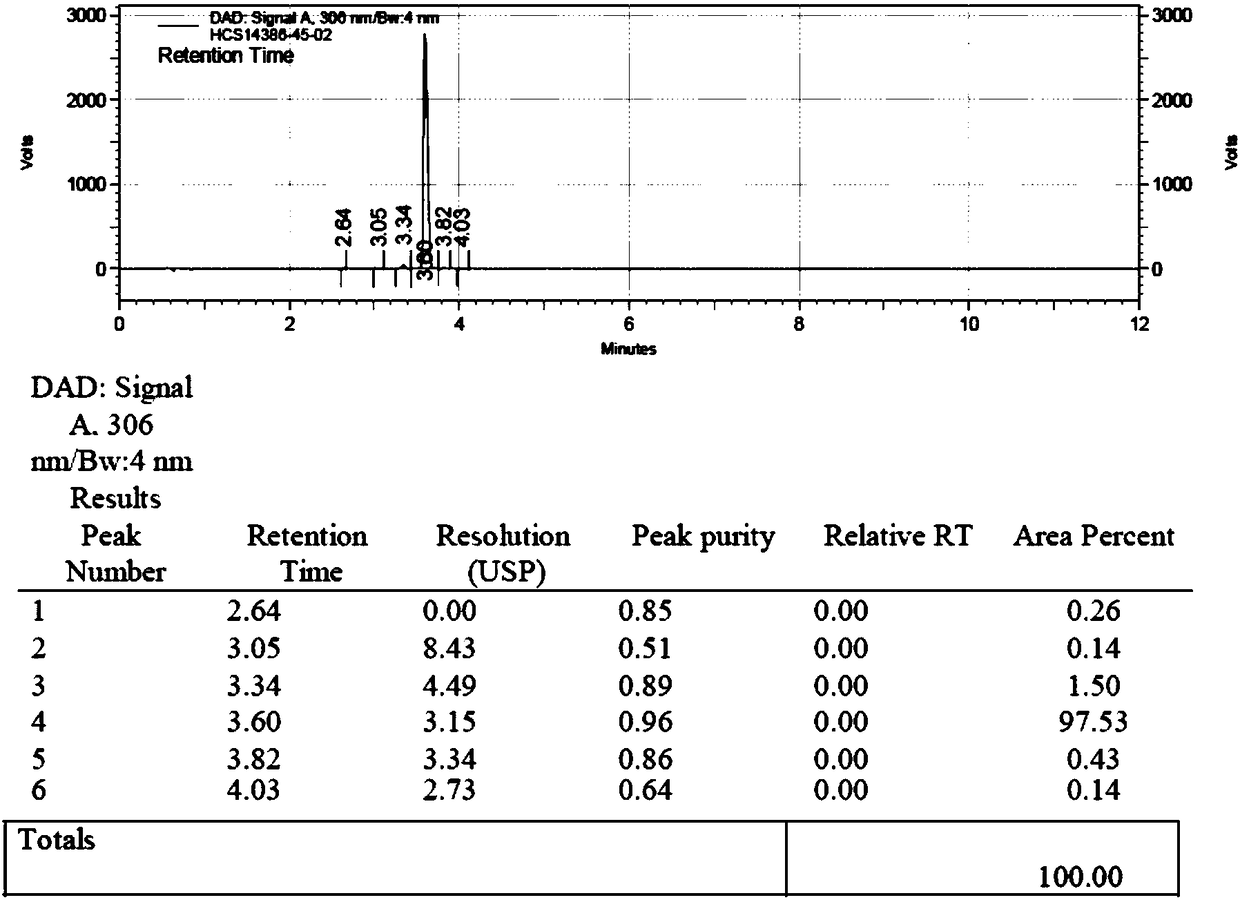
[0049] Feeding 5g of the compound shown in the formula II, 3.5g of the compound I shown in the formula II, adding 2.5 times the molar amount of the compound shown in the formula II 1-ethyl-(3-dimethylaminopropyl) carbodiimide salt Acetate and methanol 20ml, HPLC detection reaction. After the reaction (the compound shown in formula II≤0.5%), add 10ml of diisopropylethylamine, react at 30°C for 3h, and detect the reaction by thin-layer chromatography (developing agent is dichloromethane:methanol=8:1), After compound formula 1 and compound formula 2 are completely converted (the compound shown in formula 1 and the compound shown in formula 2 are both ≤0.5%) into daclatasvir, add ethyl acetate 30mL and water 20mL, extract and separate liquid, and separate to obtain organic Phase, after the organic layer was concentrated to dryness, the finished product of daclatasvir was obtained: 6.32g, the yield was 97.5%, and the HPLC purity was 99.47%.
Embodiment 3
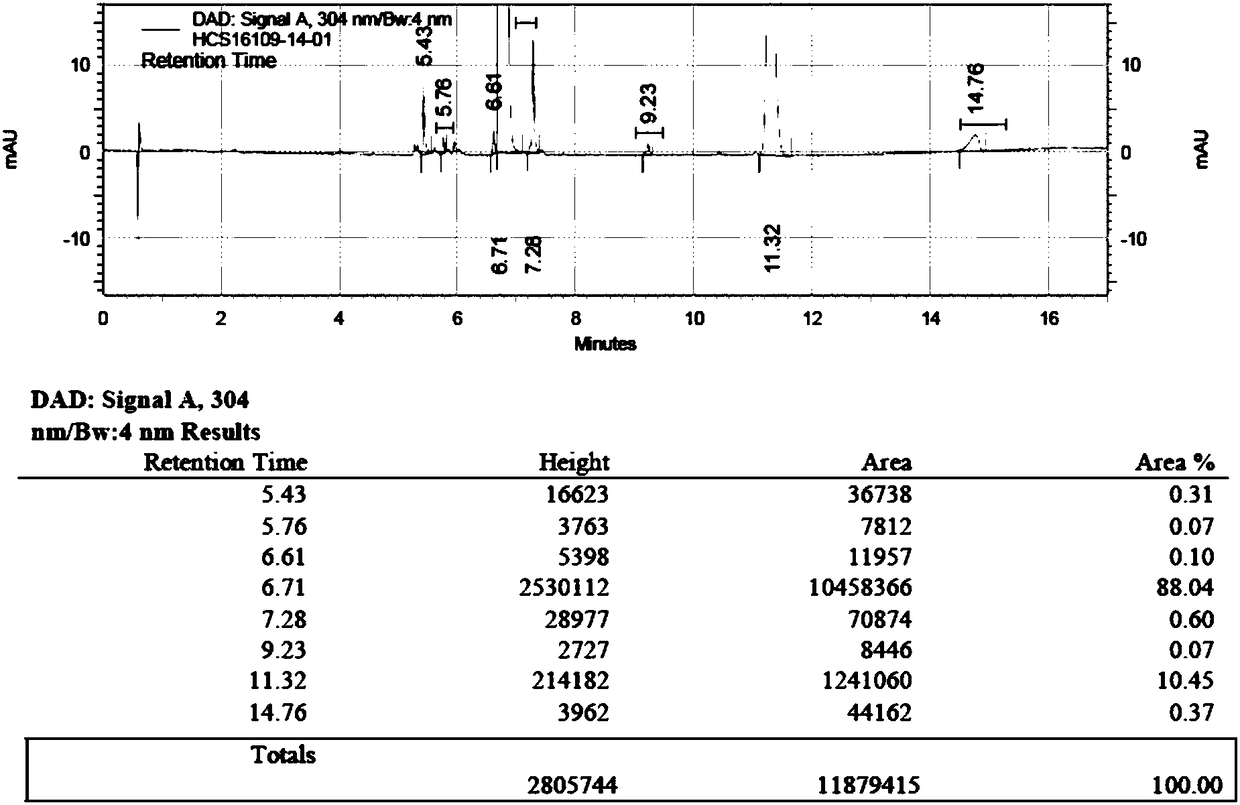
[0051]Feed intake 5g of compound shown in formula II, compound I shown in g formula II, add the 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride of 2.5 molar amounts of compound shown in formula II And acetone 20ml, HPLC detects reaction. After the reaction (the compound shown in formula II≤0.5%), add sodium hydroxide (2.1g, 6eq), react at 20°C for 1.5h, thin-layer chromatography (developing solvent is dichloromethane:methanol=8:1) Detection reaction, after compound formula 1 and compound formula 2 are completely converted (the compound shown in formula 1 and the compound shown in formula 2 are both ≤0.5%) into daclatasvir, add 30mL of isopropyl acetate and 25mL of water, extract and separate , separated to obtain the organic phase, and the organic layer was concentrated to dryness to obtain the finished product of daclatasvir: 6.41g, the yield was 99.0%, and the HPLC purity was 99.48%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com