A kind of method for separating and detecting daclatasvir hydrochloride and its optical isomers
A technology for daclatasvir and isomers, which is applied in the field of liquid chromatography for the separation and determination of daclatasvir hydrochloride and its optical isomers, can solve the problems of poor separation effect, impurity interference peak shape, and inability to adjust the mobile phase ratio. Very good improvement and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
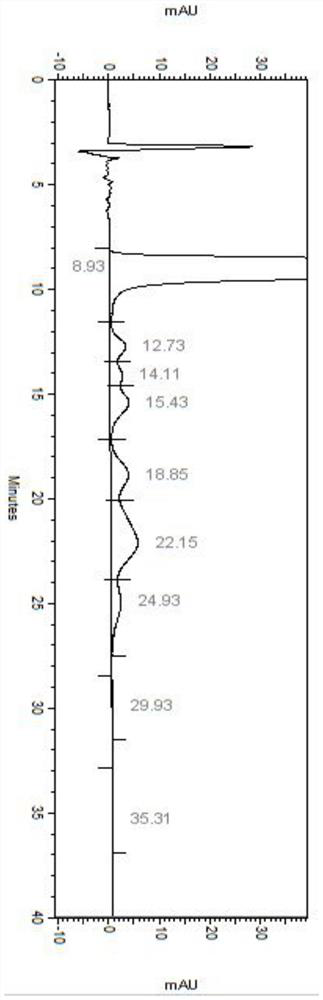
Embodiment 1
[0053] Detection conditions:
[0054] Instrument: Agilent high performance liquid chromatograph, Agilent DAD UV detector, detection wavelength: 304nm;
[0055] Chromatographic column: phenomenex Lux 5u Cellulose-1250×4.6mm chiral column;
[0056] Diluent: acetonitrile-water (1:1);
[0057] Mobile phase: mixed solution of potassium hexafluorophosphate and acetonitrile, wherein potassium hexafluorophosphate is an aqueous solution of 100 mmol / L, pH 2.0, and the mixing ratio with acetonitrile is 65:35;
[0058] Flow rate: 1.0mL / min;
[0059] Column temperature: 35℃;
[0060] Injection volume: 5μL;
[0061] Running time: 70min
[0062] Detection steps:
[0063] Take about 5 mg of each isomer impurity of daclatasvir hydrochloride, weigh it into a 5 mL volumetric flask, dissolve it with methanol and dilute to the mark to obtain the isomer impurity stock solution.
[0064] Take about 25 mg of daclatasvir hydrochloride sample, accurately weigh it into a 50 mL volumetric flask, a...
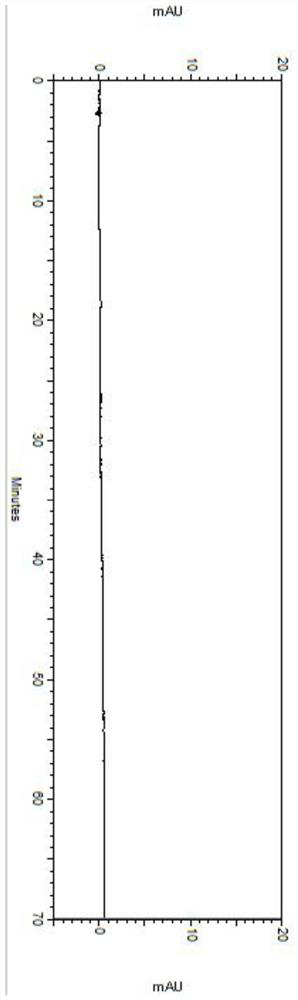
Embodiment 2
[0068] Detection conditions:
[0069] Instrument: Agilent high performance liquid chromatograph, Agilent DAD UV detector, detection wavelength: 304nm;
[0070] Chromatographic column: phenomenex Lux 5u Cellulose-1250×4.6mm chiral column;
[0071] Diluent: acetonitrile-water (1:1);
[0072] Mobile phase: mixed solution of potassium hexafluorophosphate and acetonitrile, wherein potassium hexafluorophosphate is an aqueous solution of 100 mmol / L, pH 2.0, and the mixing ratio with acetonitrile is 65:35;
[0073] Flow rate: 1.0mL / min;
[0074] Column temperature: 35℃;
[0075] Injection volume: 5μL;
[0076] Detection steps:
[0077] Take about 25 mg of daclatasvir hydrochloride sample, accurately weigh it into a 50 mL brown volumetric flask, dissolve it with a diluent and dilute it to the mark, shake well, and use it as the test solution.
[0078] Take the test solution, carry out detection and analysis according to the above conditions, record the chromatogram, see the resul...
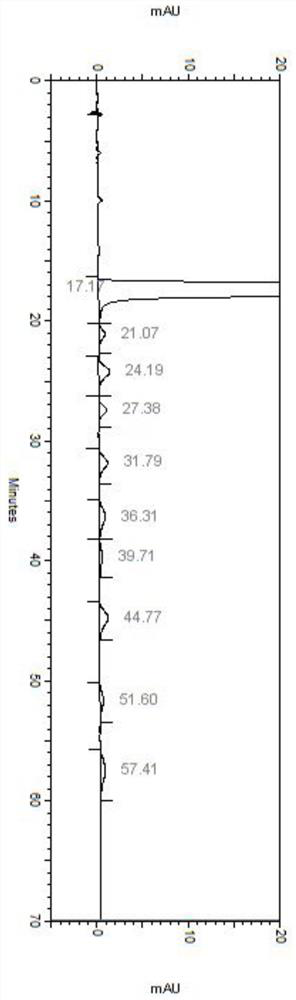
Embodiment 3
[0081] Detection conditions:
[0082] Instrument: Agilent high performance liquid chromatograph, Agilent DAD UV detector, detection wavelength: 304nm;
[0083] Chromatographic column: phenomenex Lux 5u Cellulose-1 250×4.6mm chiral column;
[0084] Diluent: acetonitrile-water (1:1);
[0085] Mobile phase: mixed solution of potassium hexafluorophosphate and acetonitrile, wherein potassium hexafluorophosphate is an aqueous solution of 100 mmol / L, pH 2.0, and the mixing ratio with acetonitrile is 65:35;
[0086] Flow rate: 1.0mL / min;
[0087] Column temperature: 30℃;
[0088] Injection volume: 5μL;
[0089] Detection steps:
[0090] Take about 5 mg of each isomer impurity of daclatasvir hydrochloride, weigh it into a 5 mL volumetric flask, dissolve it with methanol and dilute to the mark to obtain the isomer impurity stock solution.
[0091] Take about 25 mg of daclatasvir hydrochloride sample, accurately weigh it into a 50 mL volumetric flask, add 1 mL of isomer impurity st...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com