Method for separating and detecting daclatasvir hydrochloride and optical isomers thereof
A technology for daclatasvir and isomers, which is applied in the field of separation and determination of daclatasvir hydrochloride and its optical isomers by liquid chromatography, and can solve the problem of poor separation effect, impurity interference peak shape, and inability to adjust the flow phase ratio. Very good improvement and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
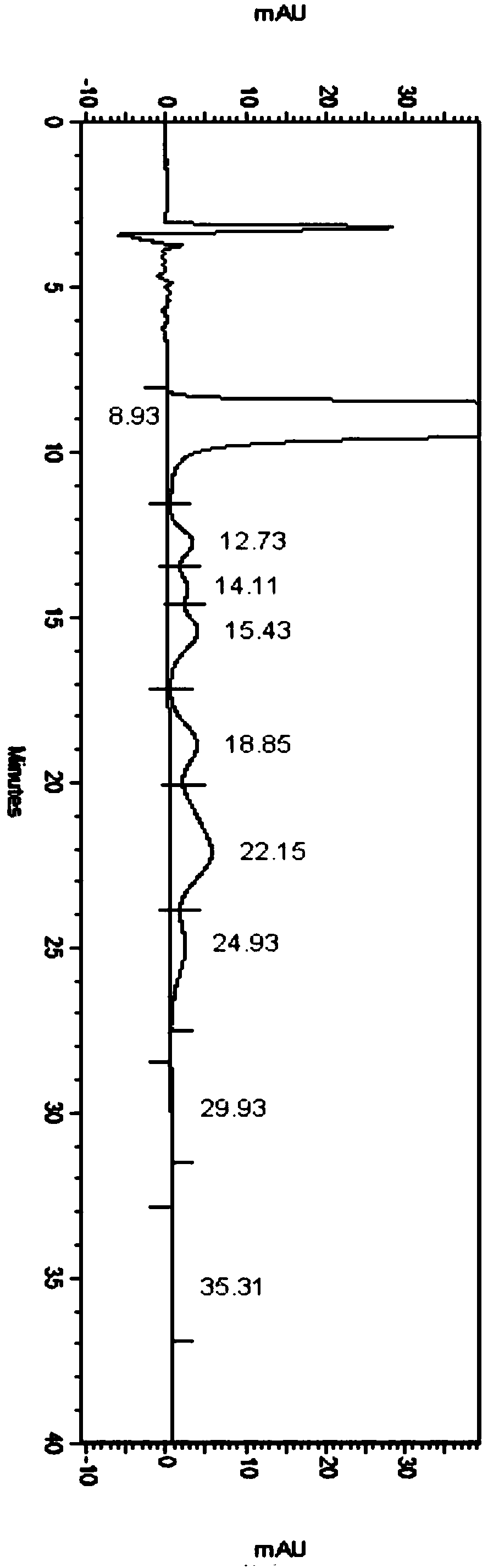
Embodiment 1
[0053] Detection conditions:
[0054] Instrument: Agilent high performance liquid chromatography, Agilent DAD ultraviolet detector, detection wavelength: 304nm;
[0055] Chromatographic column: Phenomenex Lux 5u Cellulose-1250×4.6mm chiral chromatographic column;
[0056] Diluent: acetonitrile-water (1:1);
[0057] Mobile phase: Potassium hexafluorophosphate and acetonitrile mixed solution, in which potassium hexafluorophosphate is 100mmol / L, pH 2.0 aqueous solution, and the mixing ratio of potassium hexafluorophosphate and acetonitrile is 65:35;
[0058] Flow rate: 1.0mL / min;
[0059] Column temperature: 35°C;
[0060] Injection volume: 5 μL;
[0061] Running time: 70min
[0062] Detection steps:
[0063] Take about 5 mg of the isomer impurity of daclatasvir hydrochloride, weigh it into a 5mL volumetric flask, dissolve it with methanol and dilute to the mark to obtain the isomer impurity stock solution.
[0064] Take about 25mg of daclatasvir hydrochloride sample, accu...
Embodiment 2
[0068] Detection conditions:
[0069] Instrument: Agilent high performance liquid chromatography, Agilent DAD ultraviolet detector, detection wavelength: 304nm;
[0070] Chromatographic column: Phenomenex Lux 5u Cellulose-1250×4.6mm chiral chromatographic column;
[0071] Diluent: acetonitrile-water (1:1);
[0072] Mobile phase: Potassium hexafluorophosphate and acetonitrile mixed solution, in which potassium hexafluorophosphate is 100mmol / L, pH 2.0 aqueous solution, and the mixing ratio of potassium hexafluorophosphate and acetonitrile is 65:35;
[0073] Flow rate: 1.0mL / min;
[0074] Column temperature: 35°C;
[0075] Injection volume: 5 μL;
[0076] Detection steps:
[0077] Take about 25mg of daclatasvir hydrochloride sample, accurately weigh it into a 50mL brown measuring bottle, dissolve it with a diluent and dilute to the mark, shake well, and use it as the test solution.
[0078] Get need testing solution, detect and analyze by above-mentioned condition, record c...
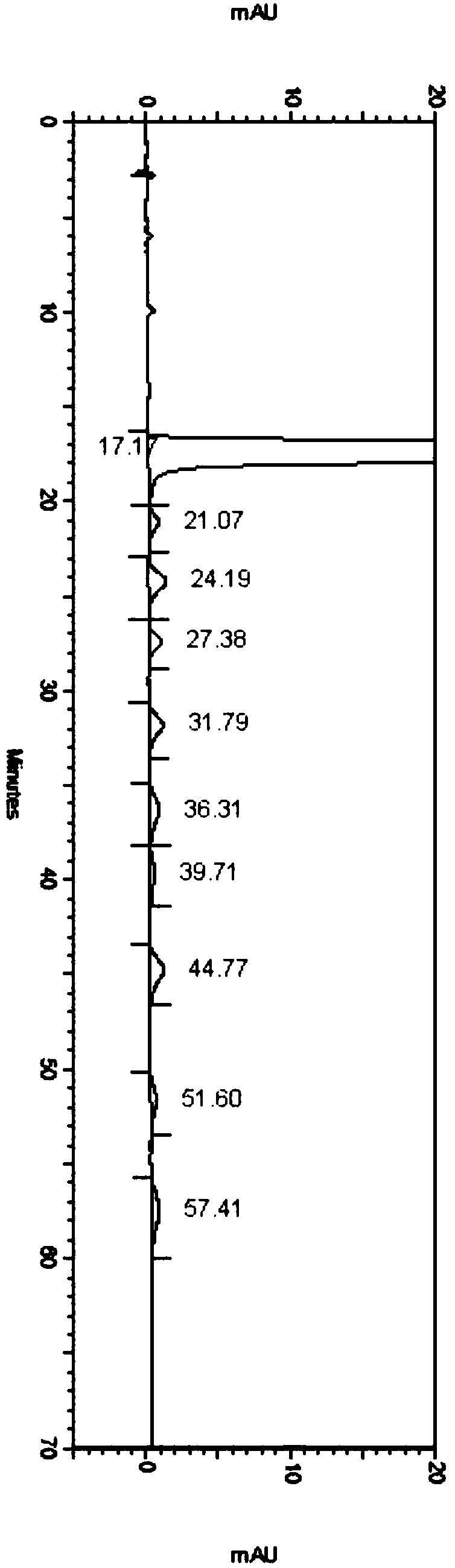
Embodiment 3
[0081] Detection conditions:
[0082] Instrument: Agilent high performance liquid chromatography, Agilent DAD ultraviolet detector, detection wavelength: 304nm;
[0083] Chromatographic column: Phenomenex Lux 5u Cellulose-1 250×4.6mm chiral chromatographic column;
[0084] Diluent: acetonitrile-water (1:1);
[0085] Mobile phase: Potassium hexafluorophosphate and acetonitrile mixed solution, in which potassium hexafluorophosphate is 100mmol / L, pH 2.0 aqueous solution, and the mixing ratio of potassium hexafluorophosphate and acetonitrile is 65:35;
[0086] Flow rate: 1.0mL / min;
[0087] Column temperature: 30°C;
[0088] Injection volume: 5 μL;
[0089] Detection steps:
[0090] Take about 5 mg of the isomer impurity of daclatasvir hydrochloride, weigh it into a 5mL volumetric flask, dissolve it with methanol and dilute to the mark to obtain the isomer impurity stock solution.
[0091] Take about 25mg of daclatasvir hydrochloride sample, accurately weigh it into a 50mL v...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com