Daclatasvir synthetic method
A synthesis method and technology of daclatasvir, applied in the field of synthesis of daclatasvir, can solve the problems of complicated operation, harsh reaction conditions, long process route and the like, and achieve the effects of simple synthesis process, short synthesis period and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
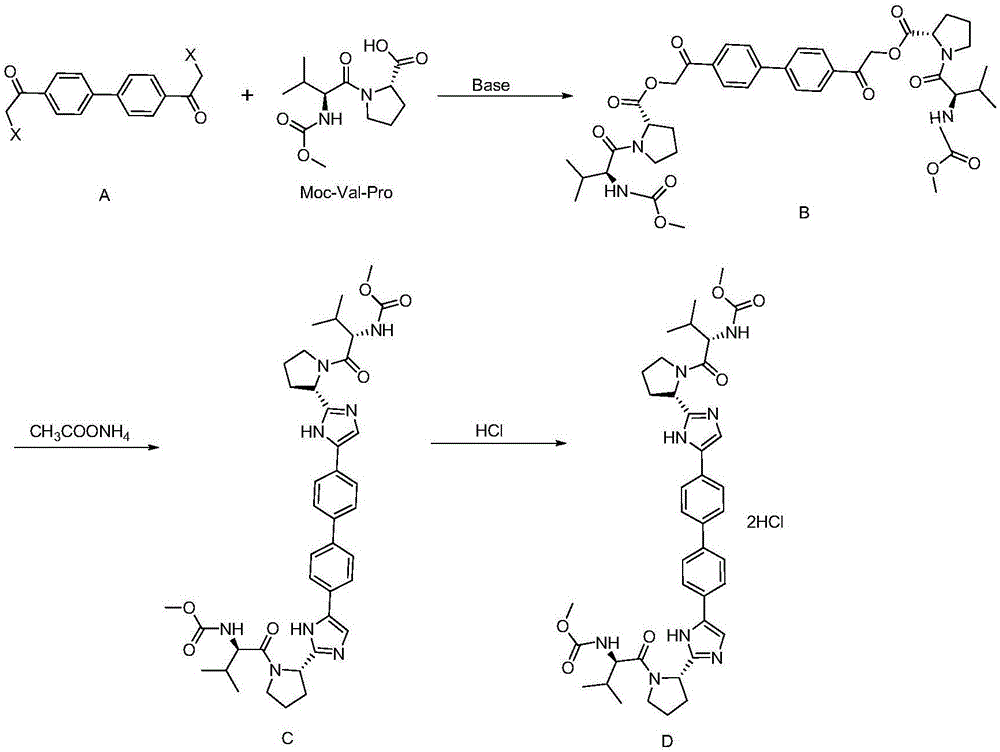
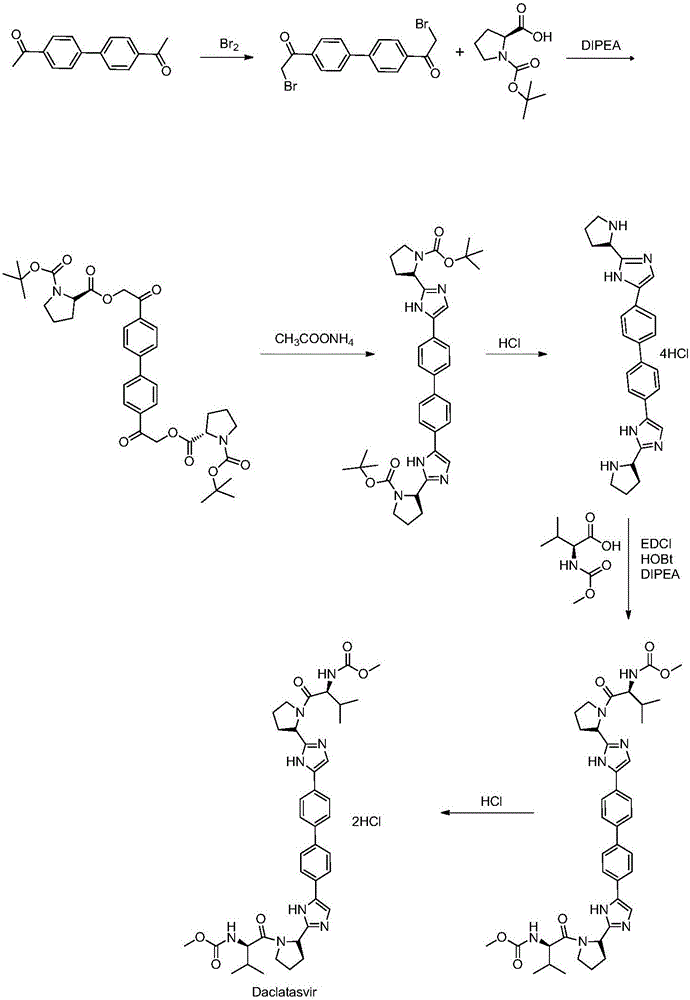
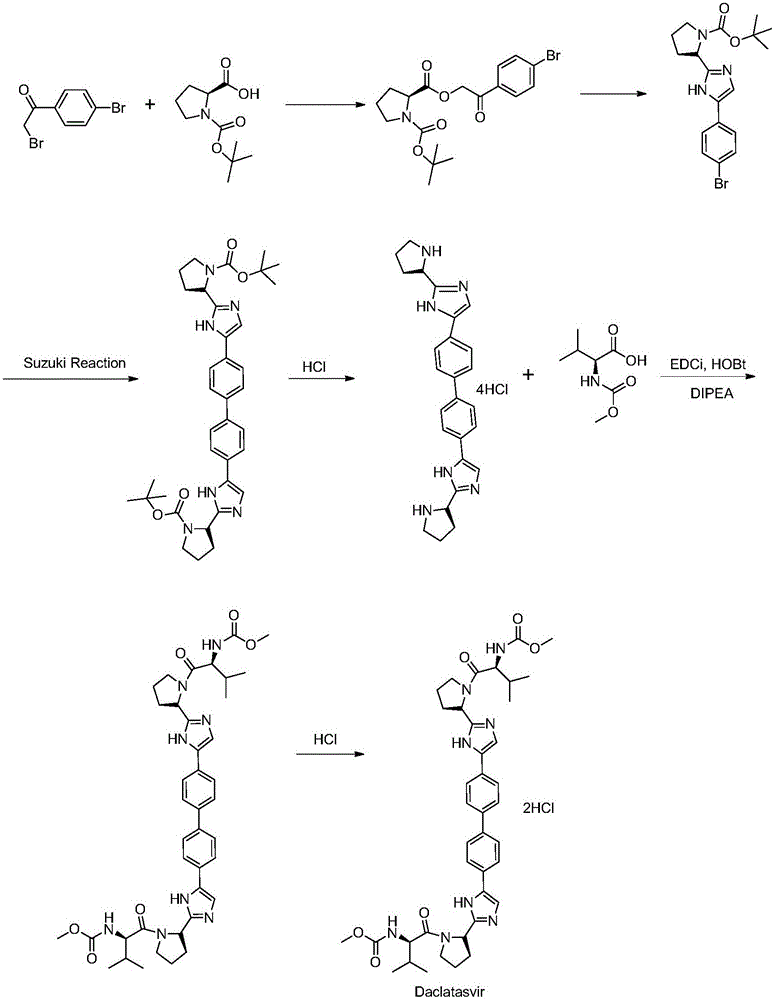
[0033] This synthetic method comprises the following steps:
[0034] Step a: Use 4,4'-bis(2-haloacetyl)biphenyl as raw material, and N-(methoxycarbonyl)-L-valine-L-proline (Moc-Val-Pro) The esterification reaction takes place in the presence of an organic solvent and a base to obtain intermediate B.
[0035] In a preferred embodiment of the present invention, the above-mentioned 4,4'-bis(2-haloacetyl)biphenyl is prepared from 4,4'-diacetylbiphenyl through a halogenation reaction. The price of 4,4'-diacetylbiphenyl is cheap and easy to obtain. The preparation of 4,4'-bis(2-haloacetyl)biphenyl through the halogenation reaction can greatly reduce the raw material of daclatasvir The cost of drug production.
[0036] In a preferred embodiment of the present invention, the halogen atom in the above halogenation reaction is Cl, Br or I, and the halogen atom is preferably Br. Further, when carrying out the bromination reaction, the brominated reagent used is selected from bromine, ...
Embodiment 1
[0054] Synthesis and purification of embodiment 1 intermediate B
[0055] 10kg of 4,4'-bis(2-bromoacetyl)biphenyl was suspended in 78kg of acetonitrile, and 17.2kg of Moc-Val-Pro and 8.15kg of N,N-diisopropylethylamine ( DIPEA), keep the temperature at 20-25 degrees for 24 hours.
[0056] After the completion of the reaction was monitored by HPLC, 40 kg of water and 30 kg of ethyl acetate were added to the reaction solution. Stir and separate the organic phase. Then wash the organic phase once with 30-40 kg of 6mol / L hydrochloric acid, then wash the organic phase once with 25 kg of 10% sodium carbonate solution, and then wash the organic phase once with 10% NaCl solution. Finally, the organic phase was dried with 10 kg of anhydrous sulfuric acid, and the solvent was removed after filtration to obtain 19.25 kg of a white solid. The yield of intermediate B was 98%, and its purity was 98.5% as detected by HPLC.
[0057] Intermediate B 1 H NMR (400MHz, DMSO-d 6 ): δ8.10(d, J...
Embodiment 2
[0058] Synthesis and purification of embodiment 2 free base C
[0059] 335kg of xylene, 19.5kg of intermediate B and 38.5kg of ammonium acetate were mixed in a reaction kettle with a water separation device, so that the pH of the reaction solution was between 4-5. Subsequently, under the protection of nitrogen, it was heated to 100° C. to allow the reaction solution to reflux. When the reaction was carried out, the water produced by the reaction was continuously removed by a water separator, and the reaction was carried out for 3 hours, and the Karl Fischer value of the reaction solution was detected to be controlled within 0.05%.
[0060] After the completion of the reaction was monitored by HPLC, after the temperature of the reaction solution was lowered to 30-35° C., 500 kg of 10% sodium bicarbonate solution and 300 kg of ethyl acetate were slowly added to the reactor, stirred and mixed, and the organic phase was separated. Then, adjust the pH of the organic phase to 3-4 w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com