High performance liquid chromatography chiral mobile phase method for separation of daclatasvir hydrochloride and five optical isomers thereof
A technology of high performance liquid chromatography and optical isomers, which is applied in the field of drug analysis, separation of daclatasvir hydrochloride and its five optical isomers by high performance liquid chromatography chiral mobile phase method, can solve the problem of high cost and applicable sexual inferiority
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
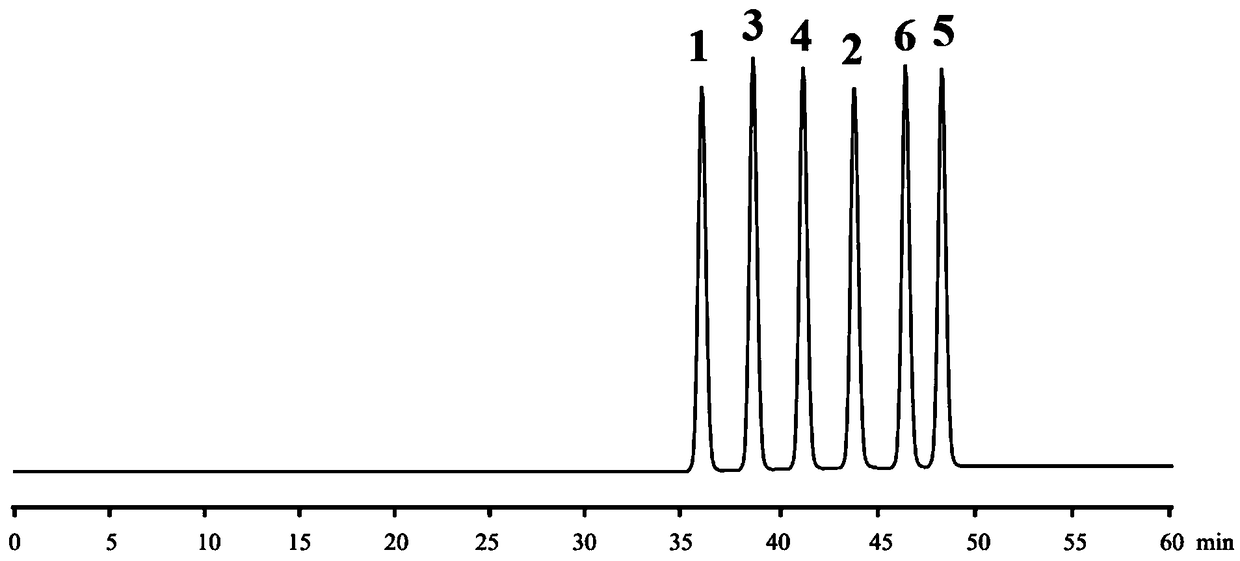
Embodiment 1
[0115] 1 (aS, bS, cS, dS type), 2 (aR, bS, cS, dS type), 3 (aS, bS, cR, dS type), 4 (aR, bS, cS, dR type), 5 ( aS, bR, cR, dS type) and 6 (aR, bR, cR, dR type) reference substances are self-made or purchased, and the purity is not less than 98%.
[0116] Preparation of mixed reference substance solution: Accurately weigh 10 mg each of 1-6 reference substances, put them in a 20ml measuring bottle, dissolve with acetonitrile and constant volume, and shake well to obtain a mixed control with a concentration of 0.5 mg / ml for 1-6 product solution.
[0117] HPLC chromatographic parameters:
[0118] Chromatograph: Waters 2695 high performance liquid chromatograph;
[0119] Chromatographic column: Agilent ZORBAX Extend-C18 (250×4.6mm, 5μm);
[0120] Mobile phase A phase: acetonitrile aqueous solution with a volume percent concentration of 20%, containing 10 mM L-aspartic acid;
[0121] Mobile phase B phase: acetonitrile aqueous solution with a concentration of 80% by volume, conta...
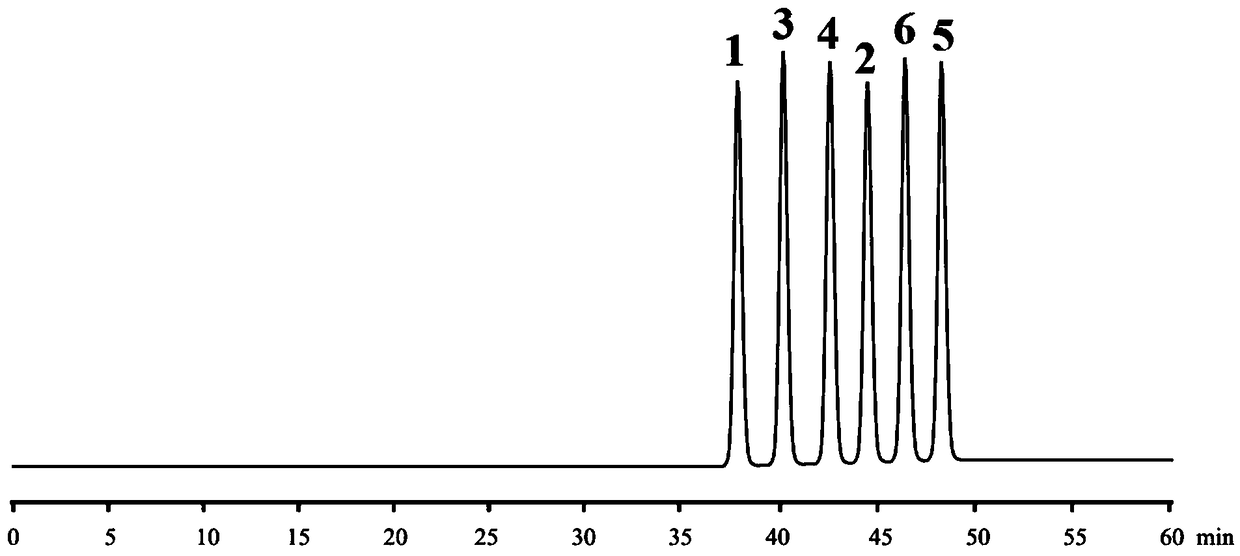
Embodiment 2
[0129] 1 (aS, bS, cS, dS type), 2 (aR, bS, cS, dS type), 3 (aS, bS, cR, dS type), 4 (aR, bS, cS, dR type), 5 ( aS, bR, cR, dS type) and 6 (aR, bR, cR, dR type) reference substances are self-made or purchased, and the purity is not less than 98%.
[0130] Preparation of mixed reference substance solution: Accurately weigh 10 mg each of 1-6 reference substances, put them in a 20ml measuring bottle, dissolve with acetonitrile and constant volume, and shake well to obtain a mixed control with a concentration of 0.5 mg / ml for 1-6 product solution.
[0131] HPLC chromatographic parameters:
[0132] Chromatograph: Waters 2695 high performance liquid chromatograph;
[0133] Chromatographic column: Agilent ZORBAX Extend-C18 (250×4.6mm, 5μm);
[0134] Mobile phase A phase: acetonitrile aqueous solution with a concentration of 20% by volume, containing 10 mM (2R, 3R)-bis-n-propyl tartaric acid;
[0135] Mobile phase B phase: acetonitrile aqueous solution with a concentration of 80% b...
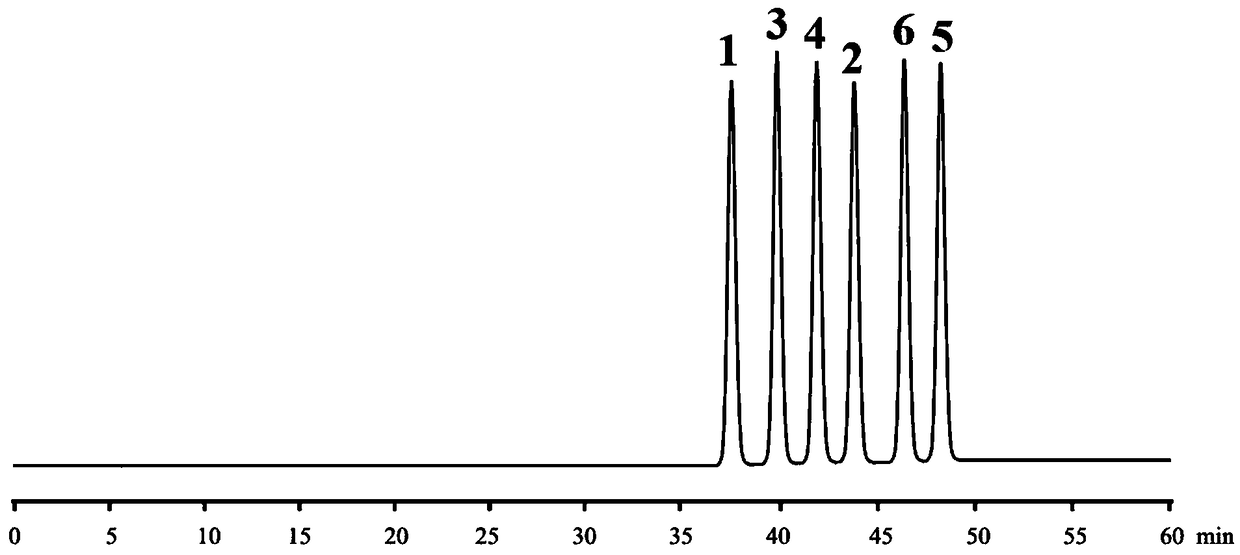
Embodiment 3
[0143]1 (aS, bS, cS, dS type), 2 (aR, bS, cS, dS type), 3 (aS, bS, cR, dS type), 4 (aR, bS, cS, dR type), 5 ( aS, bR, cR, dS type) and 6 (aR, bR, cR, dR type) reference substances are self-made or purchased, and the purity is not less than 98%.
[0144] Preparation of mixed reference substance solution: Accurately weigh 10 mg each of 1-6 reference substances, put them in a 20ml measuring bottle, dissolve with acetonitrile and constant volume, and shake well to obtain a mixed control with a concentration of 0.5 mg / ml for 1-6 product solution.
[0145] HPLC chromatographic parameters:
[0146] Chromatograph: Waters 2695 high performance liquid chromatograph;
[0147] Chromatographic column: Agilent ZORBAX Extend-C18 (250×4.6mm, 5μm);
[0148] Mobile phase A phase: acetonitrile aqueous solution with a volume percentage concentration of 20%, containing 10mM D-(+)-di-p-toluyl tartaric acid;
[0149] Mobile phase B phase: acetonitrile aqueous solution with a concentration of 80%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com