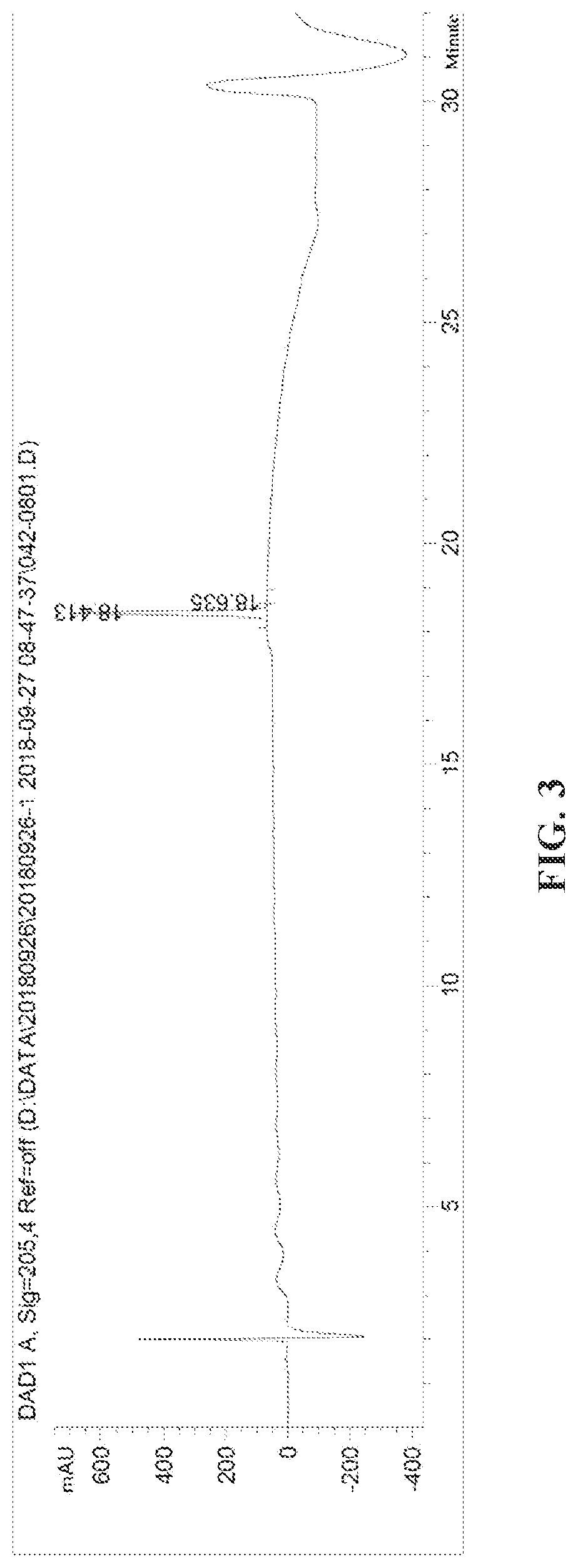
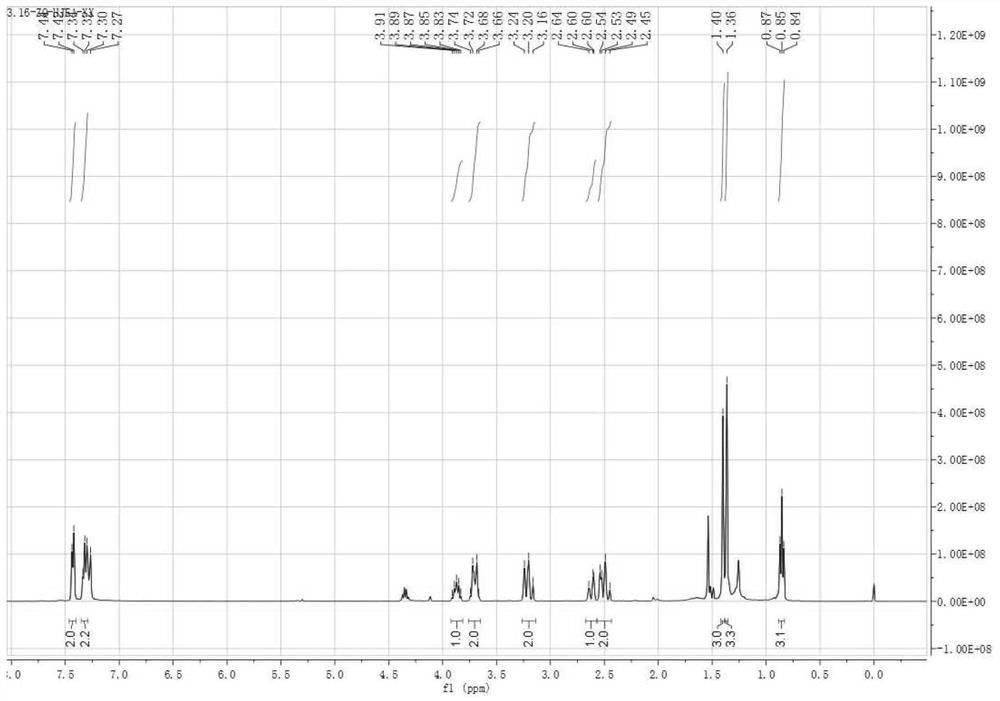
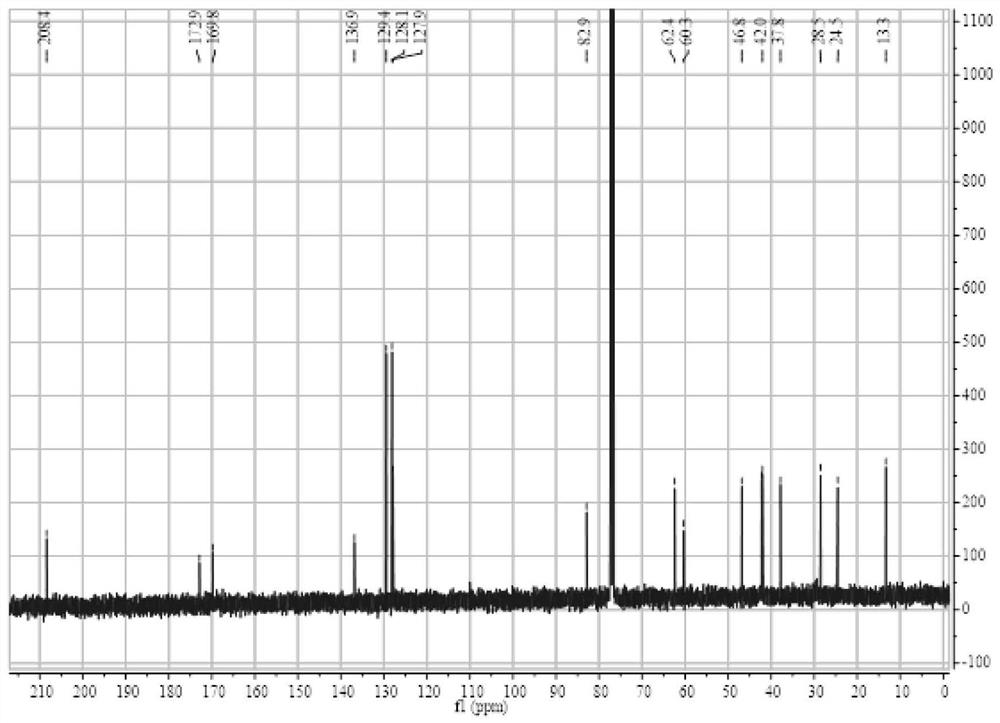
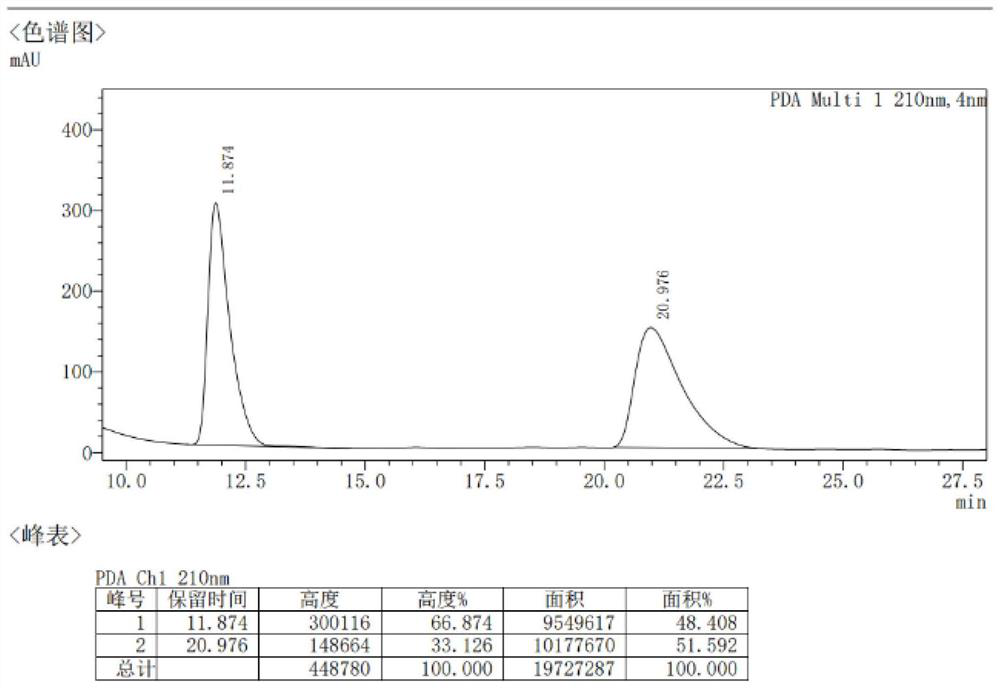
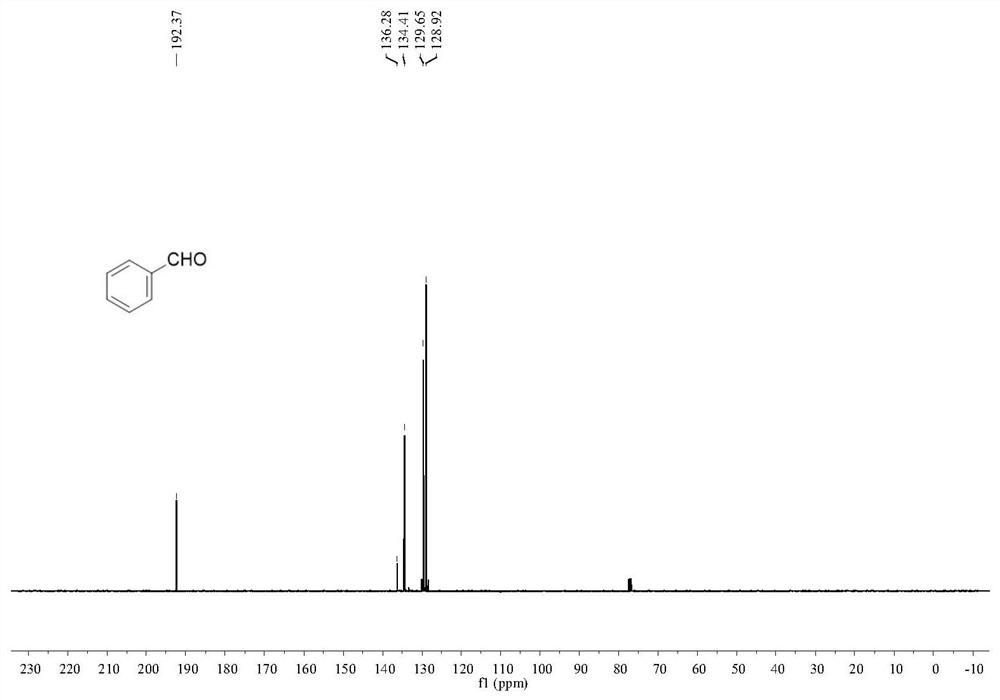
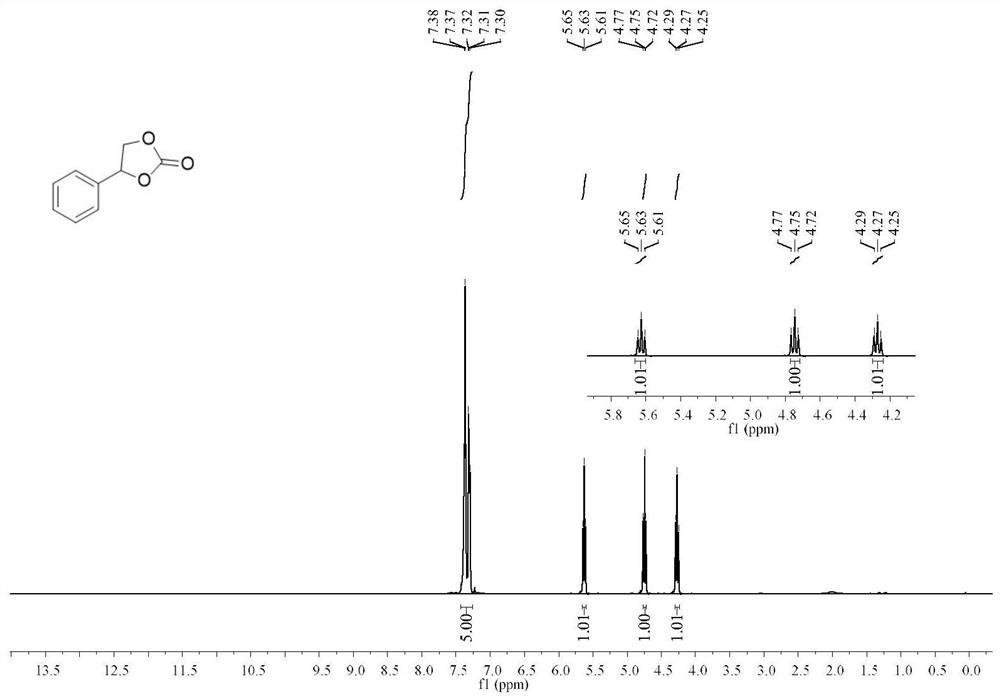
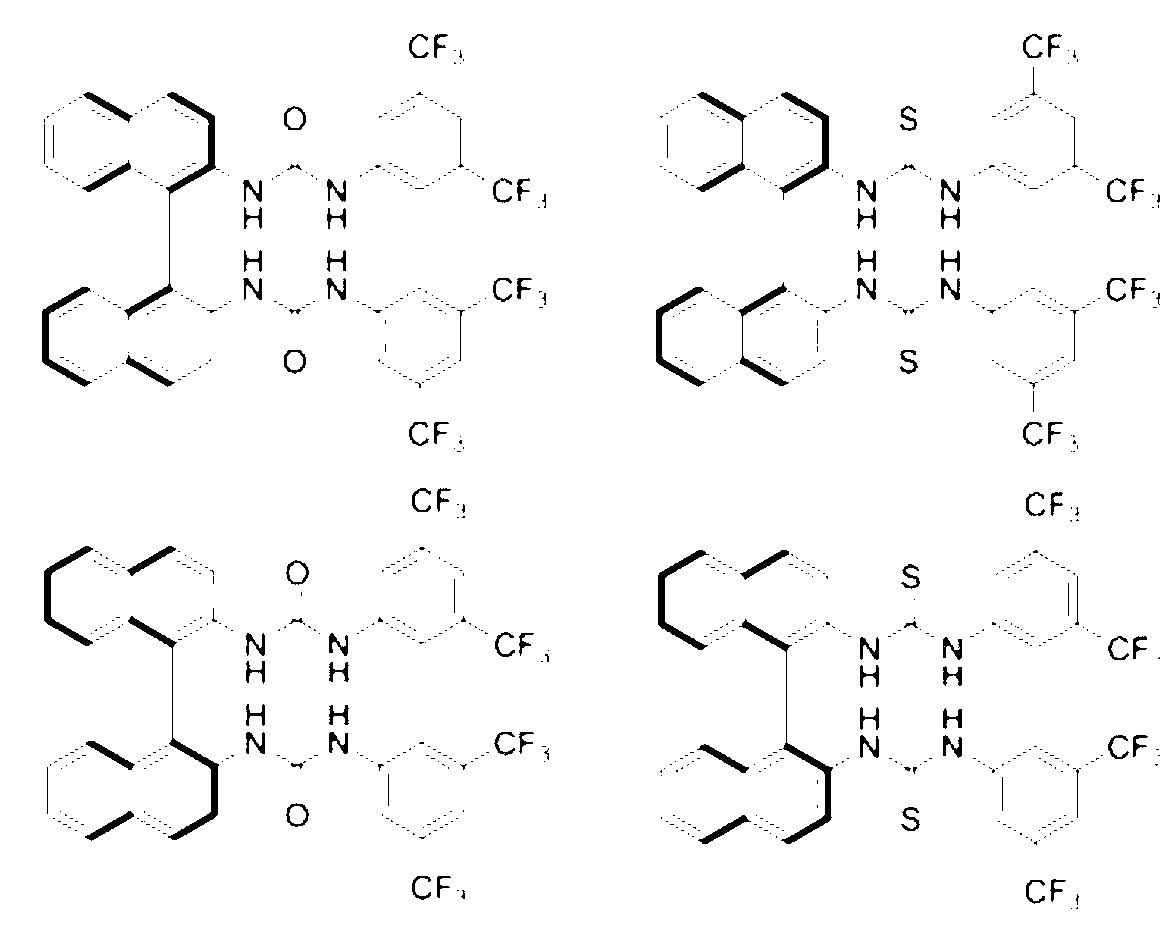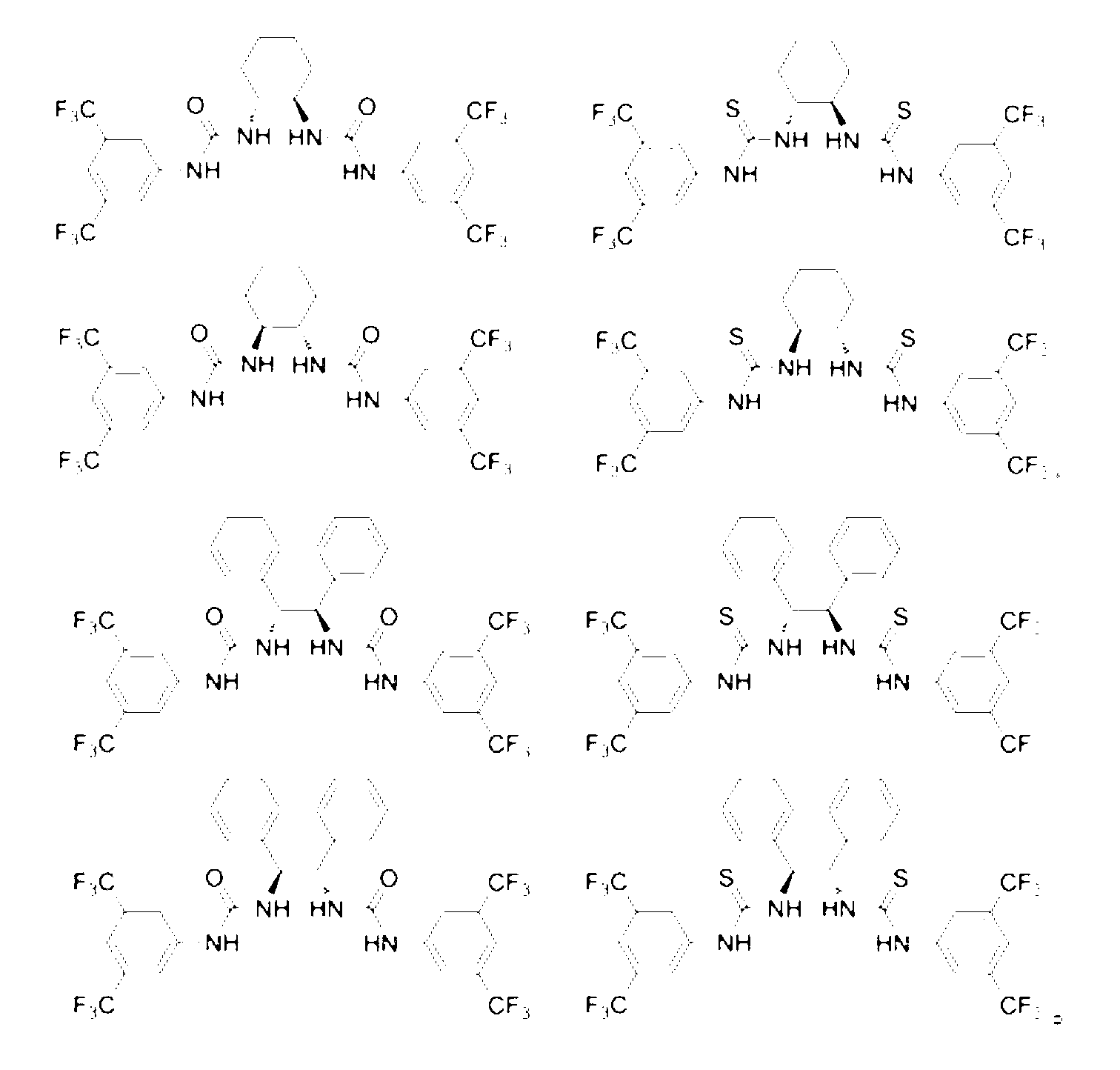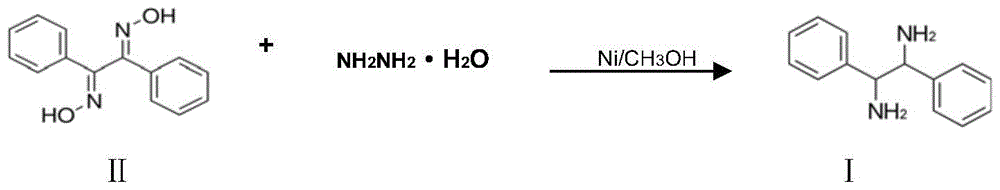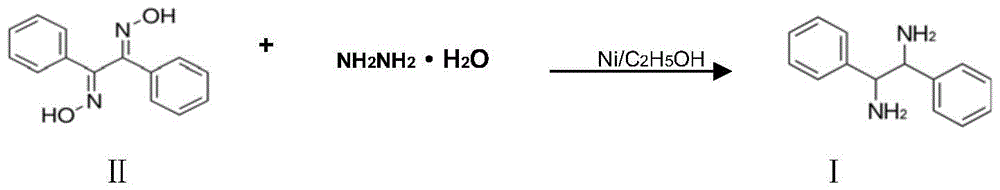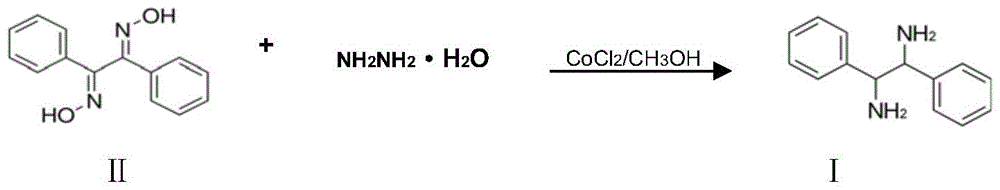Patents
Literature
39 results about "Diphenylethylenediamine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
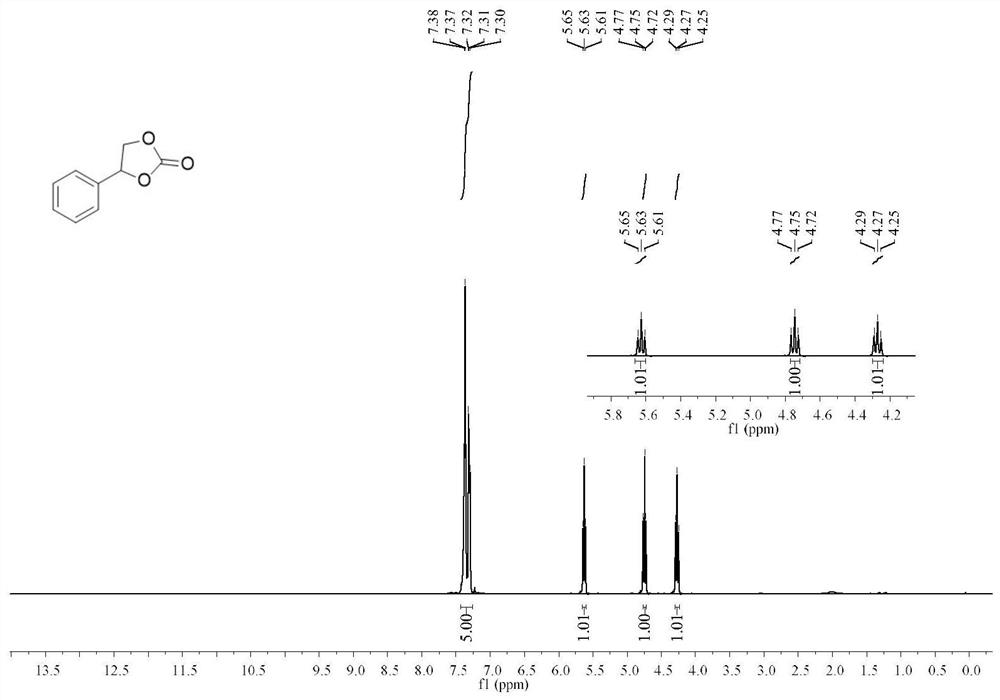
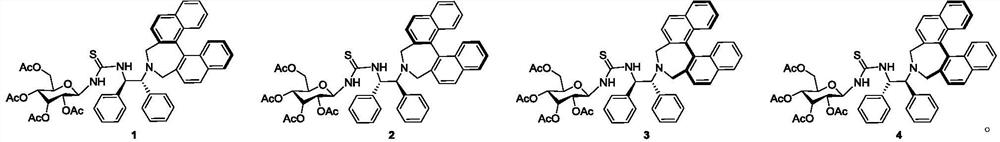
1,2-Diphenyl-1,2-ethylenediamine is an organic compound with the formula H₂NCHPhCHPhNH₂, where Ph is C₆H₅, phenyl. This diamine is a precursor to a ligand for certain homogeneous hydrogenation catalysts. It can be prepared from benzil by reductive amination.
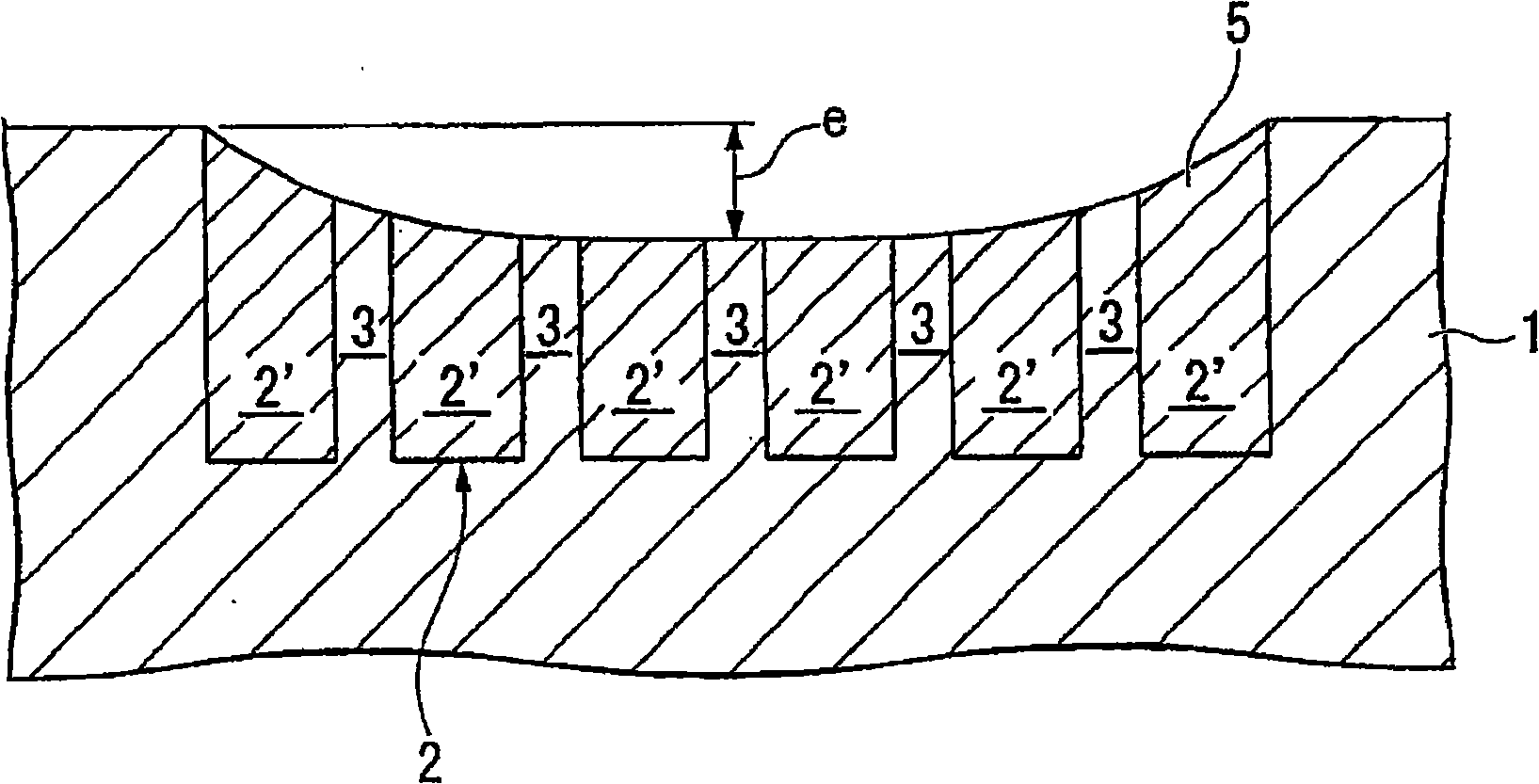
Polishing composition
InactiveUS20090289217A1Satisfactory polishing rateEasy to manufactureOther chemical processesSemiconductor/solid-state device manufacturingEthylenediamineCarboxylic acid
A polishing composition which achieves surfaces with high planarity and the reduction of corrosions in the wiring metal surface at the same time is provided.Such compositions include(A) an oxidizing agent;(B) at least one acid selected from an amino acid, a carboxylic acid of no more than 8 carbon atoms, and an inorganic acid;(C) a sulfonic acid having a concentration of 0.01% by mass or more and having an alkyl group of 8 or more carbon atoms;(D) a fatty acid having a concentration of 0.001% by mass or more and having an alkyl group of 8 or more carbon atoms; and(E) at least one compound selected from a pyridine carbonyl compound, a nonionic water-soluble polymer, 2-pyrrolidone, N-methylpyrrolidone, 1,3-dimethyl-2-imidazolidinone, gramine, adenine, N,N′-diisopropylethylenediamine, N,N′-bis(2-hydroxyethyl)ethylenediamine, N,N′-dibenzylethylenediamine, and N,N′-diphenylethylenediamine.
Owner:SHOWA DENKO KK
Polishing composition
InactiveCN101496143AFull grinding speedImprove flatnessOther chemical processesSemiconductor/solid-state device manufacturingEthylenediamineEthyl group
Disclosed is a polishing composition which realizes both high planarity and reduction of corrosion in the surface of a wiring metal. Specifically disclosed is a polishing composition containing an oxidizing agent (A), at least one or more acids (B) selected from amino acids, carboxylic acids having 8 or less carbon atoms and inorganic acids, a sulfonic acid (C) having a concentration of not less than 0.01% by mass and an alkyl group having 8 or more carbon atoms, a fatty acid (D) having a concentration of not less than 0.001% by mass and an alkyl group having 8 or more carbon atoms, and at least one or more compounds (E) selected from pyridinecarbonyl compounds, nonionic water-soluble polymers, 2-pyrrolidone, N-methylpyrrolidone, 1,3-dimethyl-2-imidazolidinone, gramine, adenine, N,N'-diisopropylethylenediamine, N,N'-bis(2-hydroxyethyl)ethylenediamine, N,N'-dibenzylethylenediamine and N,N'-diphenylethylenediamine.
Owner:SHOWA DENKO KK
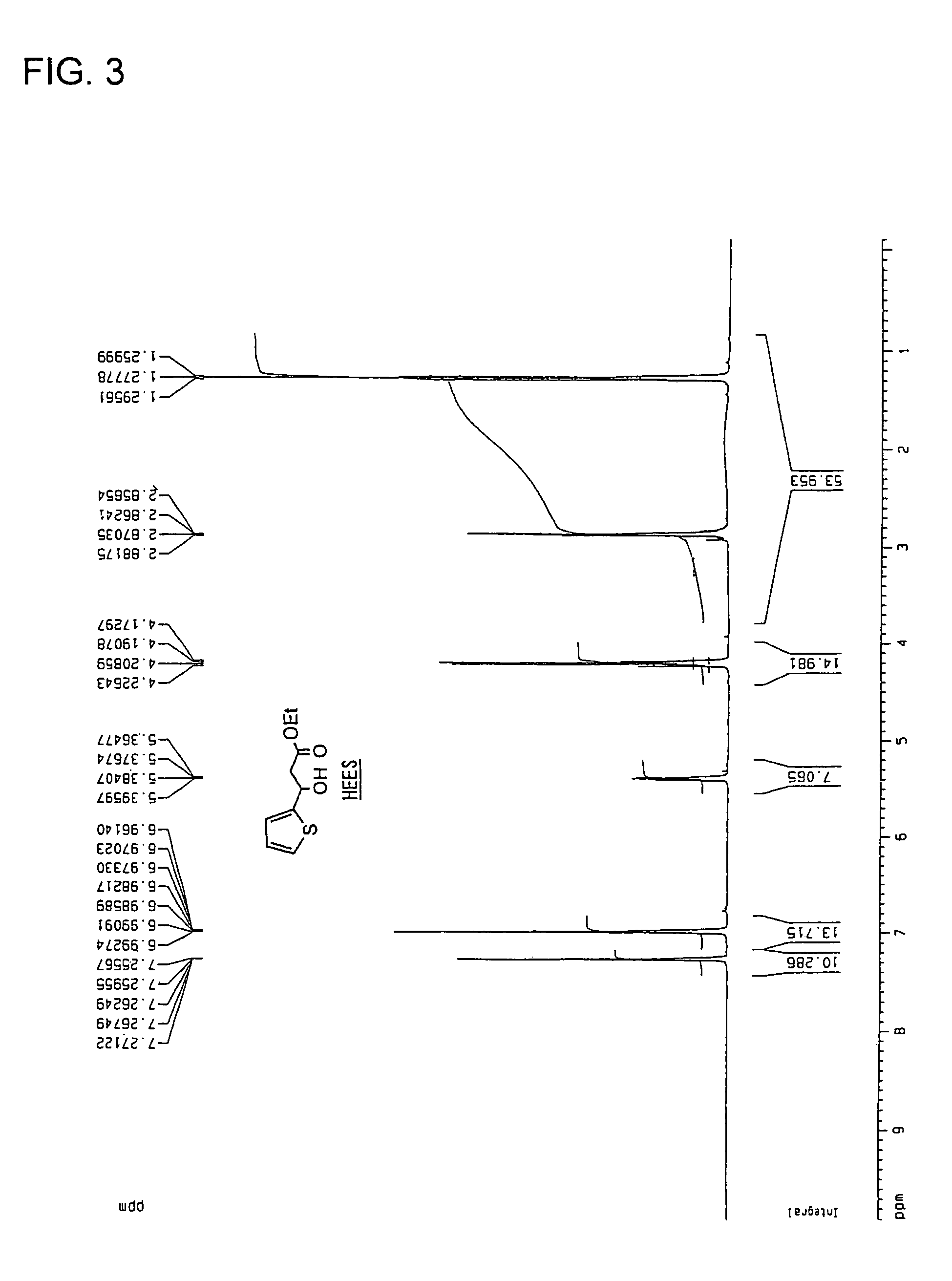
3-Hydroxy-3-(2-thienyl) propionamides and production method thereof, and production method of 3-amino-1-(2-thienyl)-1-propanols using the same
The object of the present invention is to provide 3-hydroxy-3-(2-thienyl)propionamides useful as synthesis intermediates of pharmaceutical preparations and the like and a method for obtaining optically active 3-amino-1-(2-thienyl)-1-propanols using the same with high reaction yield, high optical yield and industrially low cost. According to the present invention, 3-amino-1-(2-thienyl)-1-propanols are obtained by carrying out asymmetric reduction of a β-ketocarbonyl compound having thiophene ring in the presence of a catalyst constituted from a compound of a group VIII or IX metal in the periodic table (e.g., a ruthenium compound) and an asymmetric ligand represented by a specified optically active diamine derivative (e.g., a diphenylethylenediamine derivative), or using a cell, a treated product of said cell or the like of a microorganism, and as occasion demands, carrying out amidation of the ester group and then carrying out reduction of the amido group. (each of the substituents is as described in claim 1).
Owner:MITSUBISHI CHEM CORP
Method for measuring optical purity of chiral carboxylic acid
InactiveCN103293176AEasy to synthesizeEasy to operateAnalysis using nuclear magnetic resonanceCarboxylic acidReagent
The invention provides a method for measuring the optical purity of chiral carboxylic acid by use of a chiral chemical shift reagent. A series of chiral diamines such as 1,2-diphenylethylenediamine, cyclohexyldiamine and dithio (diurea) derivatives of binaphthyldiamine are used as chiral shift reagents, and the optical purity of the chiral carboxylic acid is quickly detected by a nuclear magnetic resonance spectrometer. The method provided by the invention has the advantages of easy synthesis of shift reagents and simplicity in operation, and is a quick, efficient, convenient and practical detection means.
Owner:FUJIAN INST OF RES ON THE STRUCTURE OF MATTER CHINESE ACAD OF SCI
Polyacid compound with metal bridging and multi-dimensional structure and application thereof
ActiveCN109776579AEasy to prepareImprove catalytic abilityWater/sewage treatment by irradiationOrganic-compounds/hydrides/coordination-complexes catalystsEthylenediamineOrganic dye
The invention discloses a polyacid compound with metal bridging and a multi-dimensional structure. The polyacid compound is obtained in the mode that ammonium molybdate, organic ligands and metal saltare subjected to a heat seal reaction in a solvent, namely an alcoholic solution, wherein the ammonium molybdate is (NH4)6Mo7O24 4H2O, the organic ligands are ethidene diamine or (1R,2R)-1,2-diphenylethylenediamine, and the metal salt is copper salt. A preparation method of the polyacid compound with metal bridging and the multi-dimensional structure is simple, the capacity of catalyzing organicdyestuff is high, and good dirt and rust removing cleaning effects are achieved. The polyacid compound can be used as a light degradation catalyst for organic pollutants in waste water, and has good application prospects in the technical fields of material science and waste water treatment.
Owner:JIAXING UNIV
Pyridine type chiral Cu(II)-Salen ligand metal organic framework crystal material as well as preparation method and application thereof
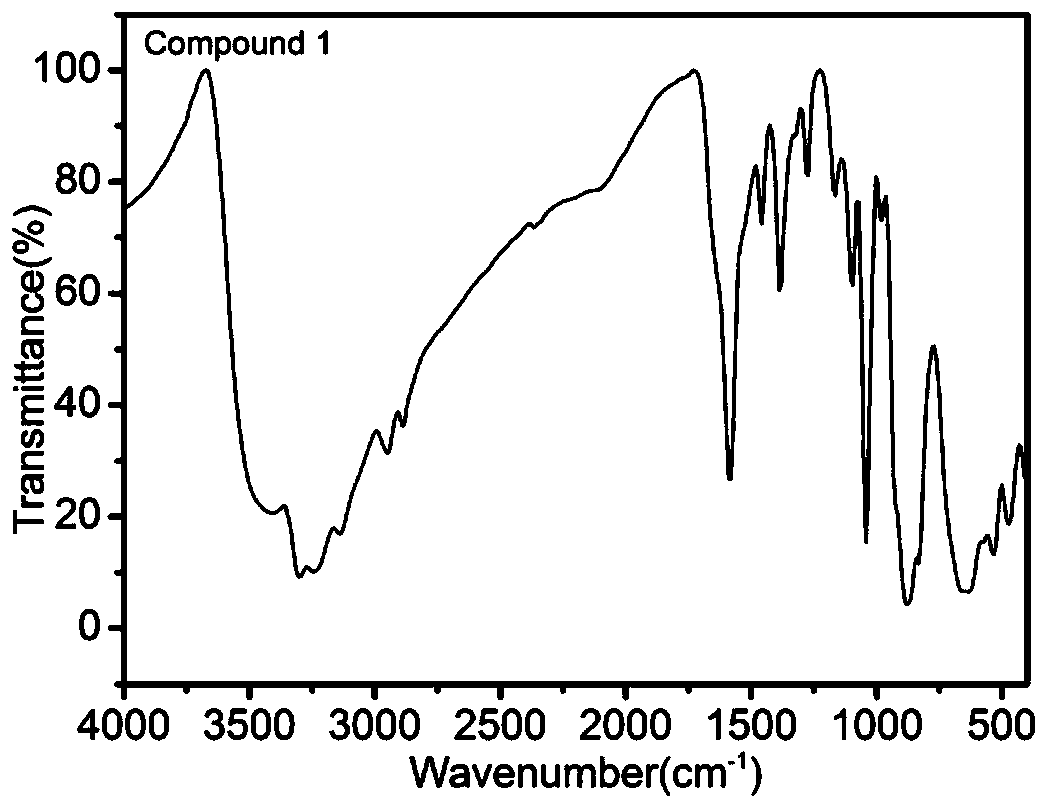
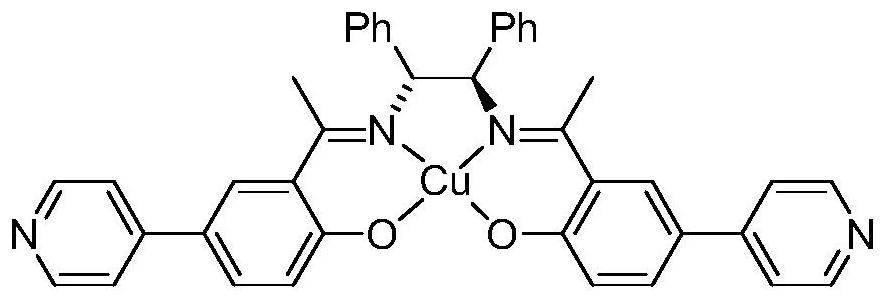
ActiveCN111690145ANovel structureUnique structureProductsOther chemical processesEthylenediamineSalen ligand
The invention relates to a pyridine type chiral Cu(II)-Salen ligand metal organic framework crystal material as well as a preparation method and application thereof. The material has the following chemical formula: {[Zn2(L)(BPDC)2].DMF.5H2O}n, wherein L is (R,R)-N,N'-bis(5-(4-pyridyl) diyl-2-hydroxyacetophenone)-1,2-diphenylethylenediamine copper (II), BPDC is a 4,4'-biphenyl dicarboxylate divalent anion, and n is the degree of polymerization. The metal organic framework crystal material provided by the invention adopts a solvothermal synthesis method, and is simple to operate, low in cost, high in yield and easy for large-scale industrial production. The prepared metal organic framework crystal material has a relatively high specific surface area (BET specific surface area is 752 m<2> / g),and the adsorption capacities of CO2 and N2 under 1atm and 273 K are 3.47 mmol / g and 0.57 mmol / g respectively. TEMPO is used as an additive, selective oxidation of benzyl alcohol is catalyzed in a water phase to generate benzaldehyde, the yield reaches 99%, the catalyst is recycled for five times with almost no activity loss, and the catalyst is a good heterogeneous catalyst.
Owner:ZUNYI MEDICAL UNIVERSITY
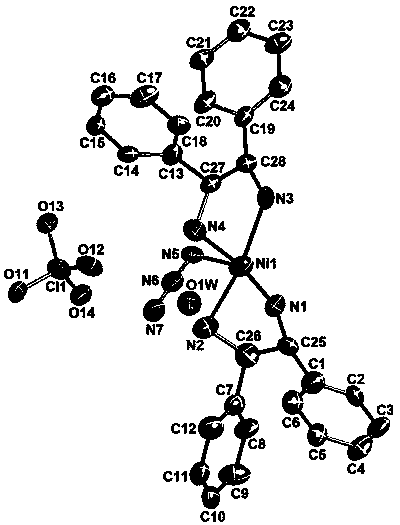
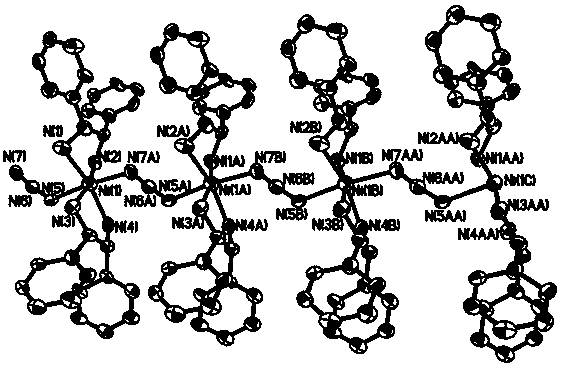
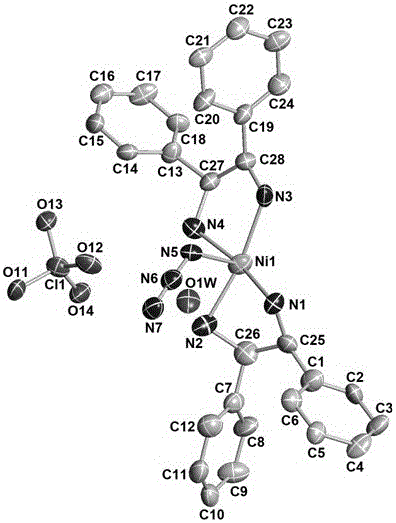
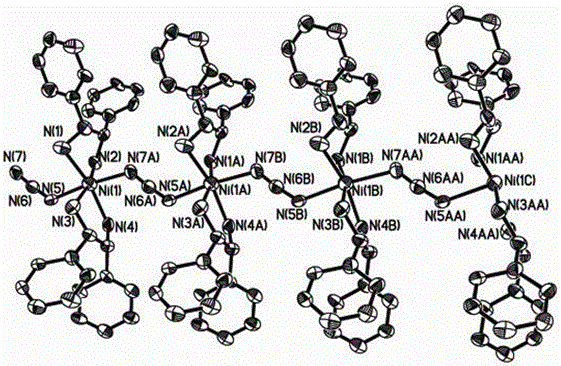
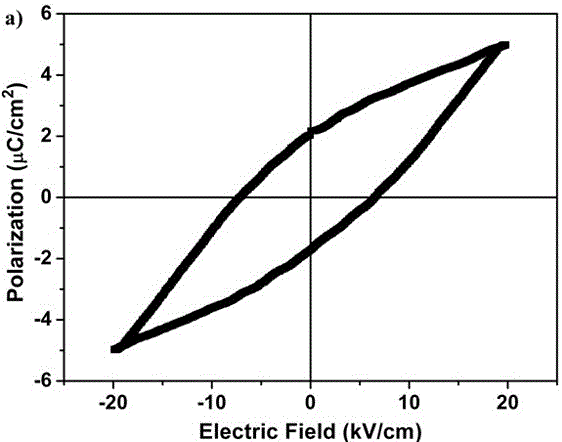
(R, R)-1,2-diphenylethylenediamine nickel azide complex and preparation method thereof
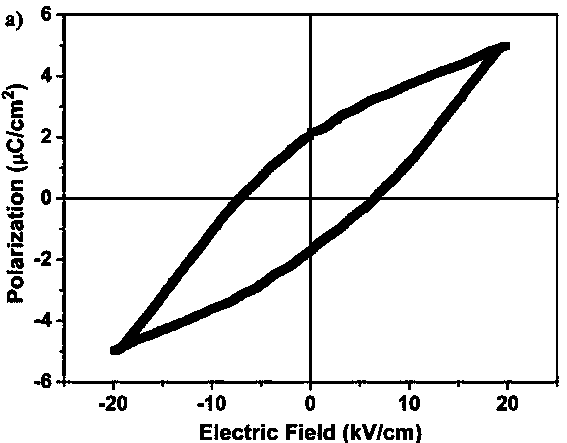
The invention discloses a (R, R)-1,2-diphenylethylenediamine nickel azide complex. The (R, R)-1,2-diphenylethylenediamine nickel azide complex has a chemical formula of [Ni(L)2(N3)] infinity ClO4.0.5H2O, wherein L is a chiral bidentate nitrogen-containing organic ligand. The preparation method comprises the following steps: placing a methanol solution as an underlayer in which nickel perchlorate and chiral bidentate nitrogen-containing organic ligand L are dissolved into a reaction vessel, adding a mixed solution of methanol and water as an intermediate buffer layer, slowly adding a water solution as an uppermost layer in which sodium azide is dissolved into the reaction vessel, standing for 5 days, filtering, washing and carrying out vacuum drying to obtain the target product. A novel one-dimensional chain nickel complex is prepared by using azide anion as a bridging ligand, the preparation process is simple, the reaction is carried out under a normal temperature and a normal pressure, the posttreatment is easily performed, and the yield is high and can reach 83%. The (R, R)-1,2-diphenylethylenediamine nickel azide complex has good ferroelectric property and has potential broad application prospects in the field of information storage as a novel ferroelectric material.
Owner:郑州轻大产业技术研究院有限公司
(1R, 2R)-1, 2-diphenylethylenediamine grafted metal-organic framework catalyst, and preparation method and applications thereof
InactiveCN103433073AImprove stabilityMild reaction conditionsOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsEnantioselective synthesisRoom temperature
The invention discloses a (1R, 2R)-1, 2-diphenylethylenediamine grafted metal-organic framework catalyst, wherein MIL-101 is used as a metal-organic framework material. The (1R, 2R)-1, 2-diphenylethylenediamine grafted metal-organic framework catalyst can be prepared by following steps: MIL-101 is subjected to vacuum dehydration for 12h at a temperature of 150 DEG C, and then is cooled to room temperature; the dehydrated MIL-101 is added into methylbenzene rapidly, and then (1R, 2R)-1, 2-diphenylethylenediamine is added into the mixture; and the mixture is subjected to refluxing and stirring for 12h at a temperature of 110 DEG C, filtered, washed with ethanol, and dried at room temperature so as to obtain the (1R, 2R)-1, 2-diphenylethylenediamine grafted metal-organic framework catalyst. The (1R, 2R)-1, 2-diphenylethylenediamine grafted metal-organic framework catalyst is friendly to the environment, and possesses high performance and stability; and reaction conditions are mild. The invention also discloses applications of the (1R, 2R)-1, 2-diphenylethylenediamine grafted metal-organic framework catalyst in asymmetric synthese of (S)-warfarin.
Owner:ZHEJIANG NORMAL UNIVERSITY
3-Hydroxy-3-(2-thienyl) propionamides and production method thereof, and production method of 3-amino-1-(2-thienyl)-1-propanols using the same
The object of the present invention is to provide 3-hydroxy-3-(2-thienyl)propionamides useful as synthesis intermediates of pharmaceutical preparations and the like and a method for obtaining optically active 3-amino-1-(2-thienyl)-1-propanols using the same with high reaction yield, high optical yield and industrially low cost.According to the present invention, 3-amino-1-(2-thienyl)-1-propanols are obtained by carrying out asymmetric reduction of a β-ketocarbonyl compound having thiophene ring in the presence of a catalyst constituted from a compound of a group VIII or IX metal in the periodic table (e.g., a ruthenium compound) and an asymmetric ligand represented by a specified optically active diamine derivative (e.g., a diphenylethylenediamine derivative), or using a cell, a treated product of said cell or the like of a microorganism, and as occasion demands, carrying out amidation of the ester group and then carrying out reduction of the amido group.(each of the substituents is as described in claim 1).
Owner:MITSUBISHI CHEM CORP
(r,r)-1,2-Diphenylethylenediamine nickel azide complex and preparation method thereof
ActiveCN104341456BHigh yieldImprove ferroelectric propertiesNickel organic compoundsSodium azideAzide
Owner:郑州轻大产业技术研究院有限公司
Novel transition metal complex and process for producing optically active alcohol
InactiveUS20060142603A1High optical purityHigh yieldRuthenium organic compoundsOrganic compound preparationDiphosphinesKetone
A novel transition metal complex, preferably a ruthenium-phosphine complex or rhodium-phosphine complex, which is effectively usable in various asymmetric syntheses and, in particular, is more effectively usable in the asymmetric hydrogenation of various ketones; and a novel process for producing an optically active alcohol with the complex. The novel transition metal complex includes a ligand obtained by introducing a diarylphosphino group into each of the 2- and 2′-positions of diphenyl ether, benzophenone, benzhydrol, or the like. It preferably further includes an optically active 1,2-diphenylethylenediamine coordinated thereto. The complex preferably is a novel diphosphine-ruthenium-optically active diamine complex or diphosphine-rhodium-optically active diamine complex. The process comprises using the complex as an asymmetric hydrogenation catalyst to conduct the asymmetric hydrogenation of a ketone compound to thereby obtain an optically active alcohol in a high optical purity and a high yield.
Owner:TAKASAGO INTERNATIONAL CORPORATION
Method for preparing brivaracetam and intermediate thereof
PendingUS20220048856A1Reduce preparation timeEasy to operateOrganic chemistry methodsBiochemical engineeringChemical compound
The present application relates to a method (I) for preparing a brivaracetam intermediate, comprising the steps of dissolving the compound represented by B-P and 1S,2S-diphenylethylenediamine in a solvent for resolution, crystallizing, filtering, and recrystallizing to obtain the compound represented by B-Q, which is then converted to the brivaracetam intermediate represented by B-R. This method can effectively resolve the compound represented by B-P. The present application also provides a method for preparing brivaracetam using the compound represented by B-R. The method can separate the effective components only through simple steps such as extraction, washing, drying, and concentration without requiring use of chiral chromatography column to separate isomers in the preparation process, and thus the separation process is simple, greatly reducing the production cost of brivaracetam.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD +1
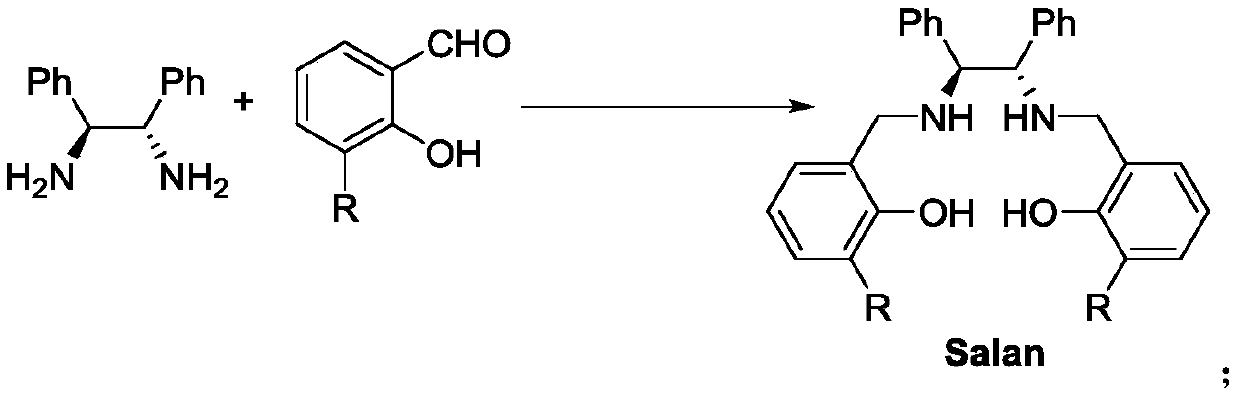
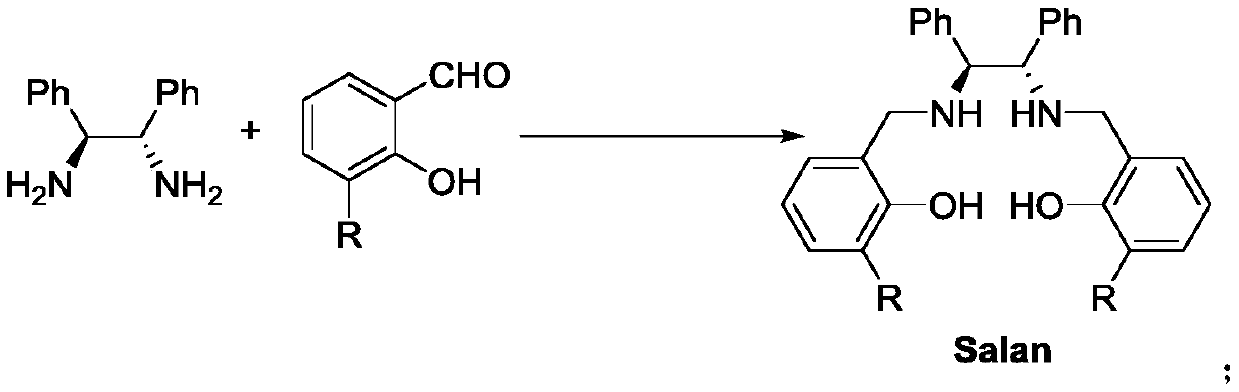
Salan ligand, metal-Salan complex and preparation method of chiral alpha-hydroxy-beta-keto ester compound
InactiveCN109776338AReduce dosageMild reaction conditionsOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsOrganic solventEnantio selectivity
The invention discloses a Salan ligand, metal-Salan complex and a preparation method of a chiral alpha-hydroxy-beta-keto ester compound. The preparation method comprises the following steps that (1S,2S)-1,2-Diphenylethylenediamine-derived Salan ligand is used as a chiral source and is coordinated with metal ions to prepare the metal-Salan complex, the prepared metal-Salan complex reacts with a substrate and an oxidant in an organic solvent to obtain the chiral alpha-hydroxy-beta-keto ester compound. The yield is up to 99% and the enantiomeric excess is up to 99% which is the highest enantioselectivity to date. The method has the advantages of mild reaction conditions, less catalyst consumption, simple operation, high enantiomeric excess and good application prospects.
Owner:UNIV OF JINAN
Cobalt complex based on chiral imidazoline as skeleton, and synthesis method and application thereof
ActiveCN114437144AHigh polymerization activityAvoid pollution, expensive and other problemsOrganic chemistry methodsCobalt organic compoundsEthylenediaminePtru catalyst
The synthesis method comprises the following steps: sequentially adding pyridylaldehyde, (1S, 2S) diphenylethylenediamine, anhydrous cobalt chloride and a solvent into a reactor, carrying out a magnetic stirring reaction in an argon atmosphere, carrying out a full reaction in a metal bath, and after the reaction is finished, carrying out a solid-liquid separation, so as to obtain the chiral imidazoline skeleton cobalt complex. And filtering, washing and drying to obtain a target product. The bidentate cobalt complex synthesized by the method disclosed by the invention is used as a catalyst for catalyzing polyisoprene and has relatively high catalytic activity.
Owner:ZHENGZHOU UNIV
High performance liquid chromatographic chiral mobile phase method for separating dakatavir hydrochloride and five optical isomers
InactiveCN108645930ALow costImprove applicabilityComponent separationSolid sorbent liquid separationTyrosineTrial and error
The invention discloses a high performance liquid chromatographic chiral mobile phase method for separating dakatavir hydrochloride and five optical isomers. The invention finds out that L-aspartic acid, (2R,3R)-bis-n-propyl tartaric acid, D-(+)-di-p-methylbenzoyl tartaric acid, (1R,2R)-(-)-N-(p-methylphenylsulfonyl)-1,2-diphenylethylenediamine or L-valyl-L-tyrosine as additives are added to a mobile phase, dacatavir hydrochloride and the five optical isomers can be effectively separated by a common C18 liquid chromatographic column, and both of the components reach baseline separation. As those skilled in the art know, a chiral mobile phase method is quite limited and only can separate limited compounds, no systematic theory exists for guiding what kind of compound to be separated by thechiral mobile phase method, and only repeated attempts with a trial-and-error method and undisciplined exploring can be used. Therefore, the method provided by the invention is not obvious to those skilled in the art and is creative as prescribed by patent laws.
Owner:覃叶枫
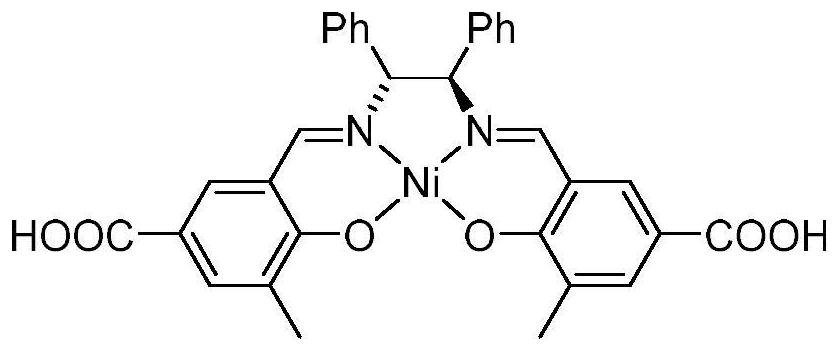
Ni (II)-Salen ligand metal organic framework crystal material and preparation method and application thereof
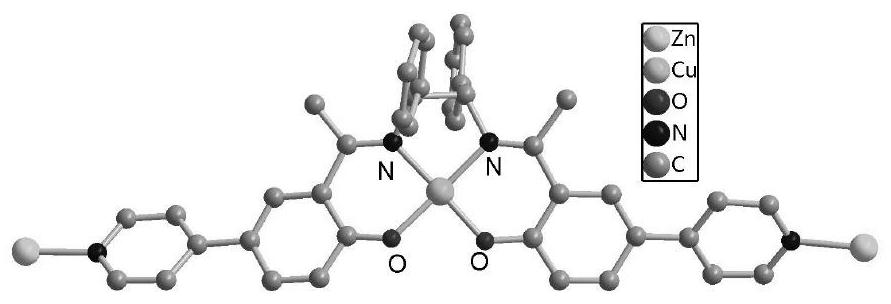
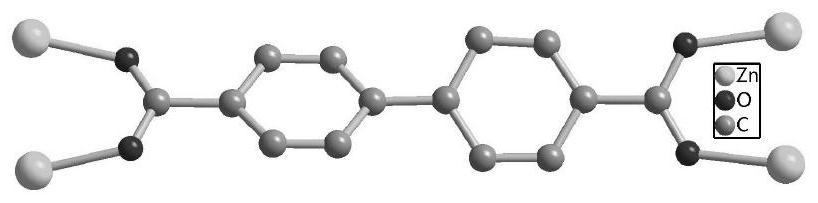
ActiveCN111732736ANovel structureUnique structureProductsOther chemical processesEthylenediamineEpoxy
The invention discloses a Ni (II)-Salen ligand metal organic framework crystal material and a preparation method and application thereof. The chemical formula of the material is {[Zn4O (L) 6]. DMF.H2O} n, wherein L is a dicarboxylic acid radical divalent anion of (R, R)-N, N '-bis (3-methyl-5-carboxyl salicylidene)-1, 2-diphenylethylenediamine nickel (II), and n is the polymerization degree. The metal organic framework crystal material is synthesized by a solvothermal synthesis method, which is simple to operate, low in cost, high in yield and easy for large-scale industrial production. The prepared metal organic framework crystal material has high thermal stability (400 DEG C), and the BET specific surface area is 228m <2> / g. And the adsorption quantity of CO2 at 273K and 1atm is 18.8 m<3> / g. In the presence of an oxidizing agent, styrene is catalyzed to be selectively oxidized to generate benzaldehyde in a water phase, the yield reaches 99%, the catalyst is recycled for five times, and the activity loss is small. In the presence of tetrabutylammonium bromide, 1 atm and 50 DEG C solvent-free catalysis is performed on epoxy styrene and CO2 to react to generate styrene carbonate, the yield is 91%, and the catalyst is recycled for five times and still keeps activity. The material is a good heterogeneous catalyst.
Owner:ZUNYI MEDICAL UNIVERSITY
Novel niacin drug salt and preparation method thereof
ActiveCN108794391BImprove solubilityHigh purityOrganic chemistry methodsOrganosolvPharmaceutical Substances
The invention relates to a novel niacin drug salt and a preparation method thereof. The salt is made from niacin and 1,2‑diphenylethylenediamine in a 2:1 molar ratio. The present invention adopts a green synthesis method of micro-liquid assisted grinding and two methods of slow volatilization at room temperature to prepare niacin-1,2-diphenylethylenediamine drug salt with high purity. The solubility of the obtained nicotinic acid drug salt is increased by 3.8 times, 6.7 times and 3.9 times in the buffer solution of pH=1.2, 4.5 and 6.8 respectively, and the crystal form is stable and does not decompose under the condition of normal temperature. The method requires only a trace amount of catalytic amount of organic solvent, has little pollution, mild reaction conditions, simple operation and high product purity.
Owner:LIAONING UNIVERSITY
Preparation method of chiral bicyclic gamma-butyrolactone compound
The invention discloses a preparation method of a chiral bicyclic gamma-butyrolactone compound, the chiral bicyclic gamma-butyrolactone compound is a main skeleton of many compounds and drug molecules and is an important active intermediate, for example, a natural product podophyllotoxin and a derivative thereof are widely applied to clinical treatment as an antitumor drug; wherein the vorapaxar sulfate serving as a marketed drug is also used as an initiative oral PAR-1 inhibitor. According to the present invention, through the double Michael addition reaction of gamma-dimethyl furanone and alpha,beta-unsaturated ketone catalyzed by (1S,2S)-1, 2-diphenylethylenediamine, so that the functional chiral bicyclic gamma-butyrolactone compound with the yield of 80%, the stereoselectivity of more than 20:1 and the higher value can be rapidly obtained through the one-step reaction. The method and the thought are provided for the synthesis of the drug active intermediate, so that synthesis difficulty of the compound in the current medicine research and development field is solved.
Owner:SHANDONG FIRST MEDICAL UNIV & SHANDONG ACADEMY OF MEDICAL SCI
Transition metal complex and process for producing optically active alcohol
InactiveUS7473793B2Easy to useHigh optical purityRuthenium organic compoundsOrganic compound preparationDiphosphinesKetone
A novel transition metal complex, preferably a ruthenium-phosphine complex or rhodium-phosphine complex, which is effectively usable in various asymmetric syntheses and, in particular, is more effectively usable in the asymmetric hydrogenation of various ketones; and a novel process for producing an optically active alcohol with the complex. The novel transition metal complex includes a ligand obtained by introducing a diarylphosphino group into each of the 2- and 2′-positions of diphenyl ether, benzophenone, benzhydrol, or the like. It preferably further includes an optically active 1,2-diphenylethylenediamine coordinated thereto. The complex preferably is a novel diphosphine-ruthenium-optically active diamine complex or diphosphine-rhodium-optically active diamine complex. The process comprises using the complex as an asymmetric hydrogenation catalyst to conduct the asymmetric hydrogenation of a ketone compound to thereby obtain an optically active alcohol in a high optical purity and a high yield.
Owner:TAKASAGO INTERNATIONAL CORPORATION
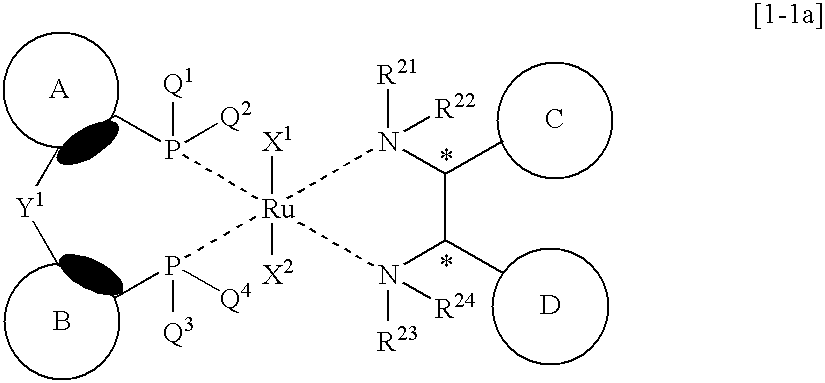
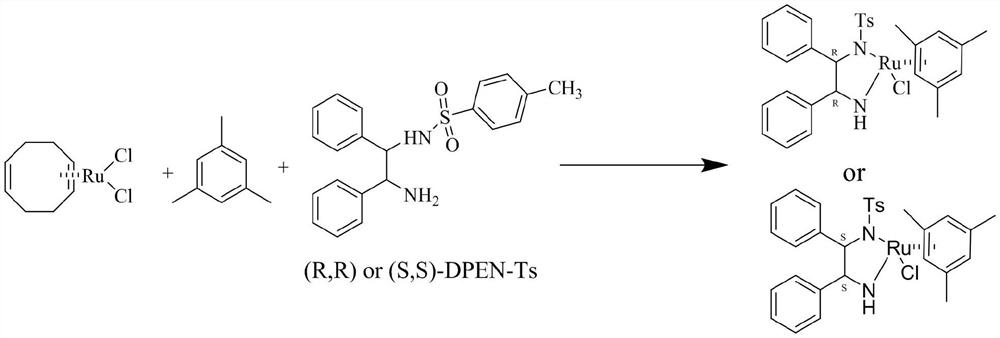
Preparation method of chiral diphenylethylenediamine ruthenium complex
InactiveCN114437141AEasy to operateHigh metal utilizationRuthenium organic compoundsOrganic chemistry methodsPtru catalystEthyl group
The invention belongs to the technical field of preparation of ruthenium noble metal catalysts in the fields of medicine synthesis, pesticides, fine chemical engineering and the like, and discloses a preparation method of a chiral diphenyl ethylenediamine ruthenium complex {[(1R, 2R) or (1S, 2S)-(-)-2-amino-1, 2-diphenyl ethyl] (p-toluenesulfonyl) amino} (mesitylene) ruthenium chloride. According to the method, the target product is obtained through one-step synthesis reaction through adjustment of the alkali reagent under the anaerobic condition and the mixed solvent system, the overall operation steps are simple and convenient, the synthesis period is short, the production efficiency is improved, the yield of the target product reaches 90.0% or above, and the purity is larger than 98.5%.
Owner:浙江微通催化新材料有限公司
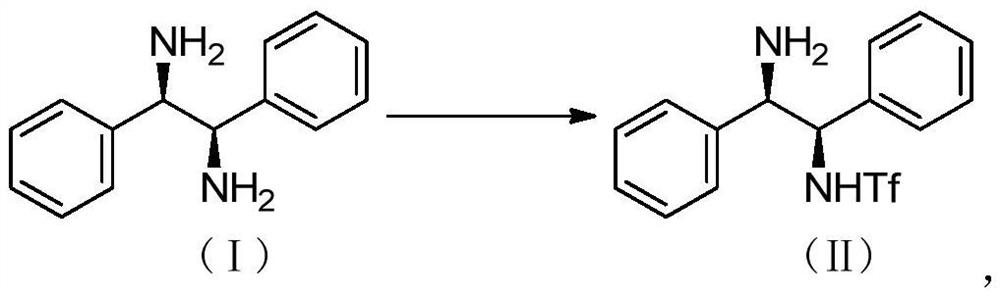
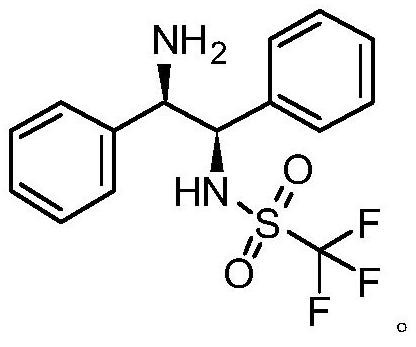
Preparation method of catalyst intermediate
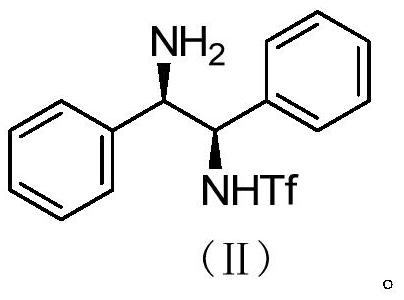
The invention relates to a preparation method of a catalyst intermediate, and in particular, relates to a preparation method of N-[(1R,2R)-2-amino-1,2-diphenyl ethyl]-1,1,1-trifluoromethanesulfonic acid amide, and belongs to the field of medicinal chemistry. The preparation method comprises the steps: salifying (1R,2R)-1,2-diphenylethylenediamine with an acid to reduce the activity of one amino group, and directly reacting the other amino group with trifluoromethanesulfonic anhydride to synthesize N-[(1R,2R)-2-amino-1,2-diphenylethyl]-1,1,1-trifluoromethanesulfonic acid amide in one step. Thepreparation method disclosed by the invention is simple in process, high in yield, low in cost, good in selectivity and friendly in reaction condition, and the ee value of the product can be well controlled and stabilized; the method has important industrial application value.
Owner:DONGGUAN HEC GENERIC DRUG R&D CO LTD +1
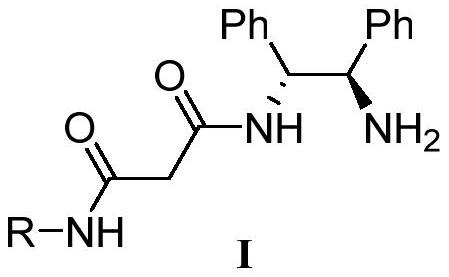
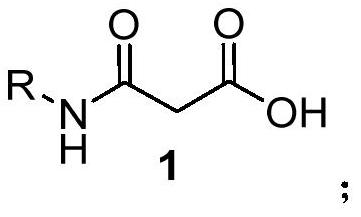
Chiral primary amine malonamide compound as well as preparation method and application thereof
ActiveCN114044742AGood activity against tobacco mosaic virusEasy to synthesizeBiocideCarbamic acid derivatives preparationMalonic acidTobacco mosaic virus
The invention relates to a chiral primary amine malonamide compound as well as a preparation method and application thereof. The structural formula of the compound is shown in the specification. In the preparation method, (1R,2R)-1,2-diphenylethylenediamine reacts with a malonic acid derivative to obtain the chiral primary amine malonamide compound containing a malonamide structural unit. The chiral primary amine malonamide compound obtained by the preparation method disclosed by the invention has very good tobacco mosaic virus resisting activity.
Owner:HEBEI UNIV OF TECH
Porous chiral organic polymer catalyst and preparation method thereof
ActiveCN109894148ACurb churnOvercome the difficult problem of separation and recoveryOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsFixed bedHydrosilylation
The invention discloses a porous chiral organic polymer heterogeneous catalyst based on a chiral 1,2-diphenylethylenediamine metal complex, and a preparation method thereof and belongs to the field ofsynthesis and application of functional materials. The heterogeneous catalyst takes chiral 1,2-diphenylethylenediamine and the coordinated metal thereof as active species, and can be obtained throughtwo methods: method 1, carrying out copolymerization between alkylene chiral 1,2-diphenylethylenediamine and another monomer to obtain a porous organic polymer, and coordinating with the metal complex; method 2, firstly coordinating alkylene chiral 1,2-diphenylethylenediamine with the metal complex, and copolymerizing with another monomer. The heterogeneous catalyst of this type is applied to reactors of a fixed bed, a slurry bed, a trickle bed and the like. According to the heterogeneous catalyst provided by the invention, the catalyst is applied to reaction technologies of chiral hydrogenation, chiral hydrogen transfer and chiral hydrosilylation of aromatic ketone or aromatic amine, and the advantages of high-activity and high selectivity of the homogeneous phase metal complex, and easyseparation of the heterogeneous catalyst are remained.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Chiral tridentate imine P, N, N-ligand, preparation method and application of chiral tridentate imine P, N, N-ligand in Cu-catalyzed asymmetric propargyl conversion
ActiveCN114539327AFavor secondary effectsEasy to prepareOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystAcyl group
The invention provides a chiral tridentate imine P, N, N-ligand, a preparation method and an application of the chiral tridentate imine P, N, N-ligand in Cu-catalyzed asymmetric propargyl conversion, the chiral tridentate imine P, N, N-ligand has stable properties, has good tolerance to air and humidity and contains N-H functional groups, and the chiral tridentate imine P, N, N-ligand is prepared from chiral ferrocenephosphine-1, 2, 3, 4-tetramethyl-1, 3, 4-tetramethyl-1, 3, 4-tetramethyl-1, 3, 4-tetramethyl-1, 3, 4-tetramethyl-1, 3, 4-tetramethyl-1, 3, 4 A 1, 2-diphenylethylenediamine compound and a 2-acyl pyridine compound are used as raw materials, and one-step reaction is performed under mild conditions under the action of a dehydrating agent. The catalyst composed of the novel chiral tridentate imine P, N, N-ligand and a Cu metal precursor has excellent catalytic activity and stereoselectivity in an asymmetric propargyl conversion reaction.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
A method for measuring the optical purity of chiral carboxylic acid
InactiveCN103293176BEasy to synthesizeEasy to operateAnalysis using nuclear magnetic resonanceCarboxylic acidReagent
The invention provides a method for measuring the optical purity of chiral carboxylic acid by use of a chiral chemical shift reagent. A series of chiral diamines such as 1,2-diphenylethylenediamine, cyclohexyldiamine and dithio (diurea) derivatives of binaphthyldiamine are used as chiral shift reagents, and the optical purity of the chiral carboxylic acid is quickly detected by a nuclear magnetic resonance spectrometer. The method provided by the invention has the advantages of easy synthesis of shift reagents and simplicity in operation, and is a quick, efficient, convenient and practical detection means.
Owner:FUJIAN INST OF RES ON THE STRUCTURE OF MATTER CHINESE ACAD OF SCI
Waterproof silicone rubber compound and preparation method thereof
InactiveCN104312161AImprove hydrolysis resistanceImprove mechanical propertiesMethacrylatePolymer science
The invention discloses a waterproof silicone rubber compound and a preparation method thereof. The waterproof silicone rubber compound consists of the following raw materials by weight: 60-80 parts of methyl vinyl phenyl polysiloxane rubber, 30-50 parts of polyethersulfone, 2-3 parts of 1,1-Di(tert-butylperoxy) cyclohexane, 4-8 parts of tetraethyl orthosilicate, 2.5-4.5 parts of zinc methacrylate, 2-3 parts of bismaleimide, 5-10 parts of spindle oil, 12-18 parts of sub spherical silica powder, 3-5 parts of dipropylene glycol, 10-15 parts of magnesite powder, 1-2 parts of isopropyl tri(dioctylpyrophosphate) titanate, 3-6 parts of Fischer Tropsch wax, 1-2 parts of 2-amino propane, 2-3 parts of N,N'-diphenylethylenediamine and 3-4 parts of an auxiliary agent. The silicone rubber compound provided by the invention has excellent hydrolysis resistance, can resist hot water or steam of 150 to 160 DEG C while still keeping high mechanical properties, also has good heat resistance, creep resistance, impact resistance and chemical corrosion resistance, and can greatly expand the scope of application.
Owner:天长市荣盛有机硅科技有限公司
A kind of preparation method of (±)‑1,2‑diphenylethylenediamine
ActiveCN105218380BSolve the dangerEasy to operatePreparation by N-O/N-N bondsHydrazine compoundSolvent
The invention discloses a preparation method for (+ / -)-1,2-diphenylethanediamine. The (+ / -)-1,2-diphenylethanediamine in the invention is prepared from 1,2-dibenzoyl dioxime by catalytic reduction in a polar solvent at 20 DEG C to 100 DEG C with one selected from the system consisting of hydrazine hydrate, formic acid, sodium formate, ammonium formate, and ammonium formate-triethylamine as a hydrogen source, and with raney nickel as a catalyst and activated carbon as a cocatalyst. The preparation method provided by the invention has the advantages of simple and wide raw material sources, low price, safe production process, short process flow, mild reaction conditions, low cost, environment friendliness, etc., and is suitable for industrial production.
Owner:LIANYUNGANG CHIRAL CHEM CHINA CO LTD
A Ni(ii)-salen ligand metal-organic framework crystal material and its preparation method and application
ActiveCN111732736BNovel structureUnique structureProductsOther chemical processesEthylenediamineSalen ligand
Ni(II)-Salen ligand metal-organic framework crystal material and its preparation method and application. The chemical formula of the material: {[Zn 4 O(L) 6 ]·DMF·H 2 O} n , where L is the dicarboxylate dicarboxylate of (R,R)-N,N'-bis(3-methyl-5-carboxysalicylidene)-1,2-diphenylethylenediamine nickel(II) Valence anion, n is the degree of polymerization. The metal organic framework crystal material adopts a solvothermal synthesis method, has simple operation, low cost, high yield, and is easy for large-scale industrial production. The prepared MOF crystalline material has high thermal stability (400°C), and the BET specific surface area is 228m 2 / g. For CO at 273K, 1atm 2 The adsorption capacity is 18.8m 3 / g. In the presence of an oxidizing agent, the selective oxidation of styrene is catalyzed in an aqueous phase to generate benzaldehyde with a yield of 99%. The catalyst is recycled five times with little loss of activity. In the presence of tetrabutylammonium bromide, 1 atm, 50 ℃ solvent-free catalysis of epoxy styrene and CO 2 The reaction produces styrene carbonate with a yield of 91%. The catalyst is recycled five times and still maintains its activity. This material is a good heterogeneous catalyst.
Owner:ZUNYI MEDICAL UNIVERSITY
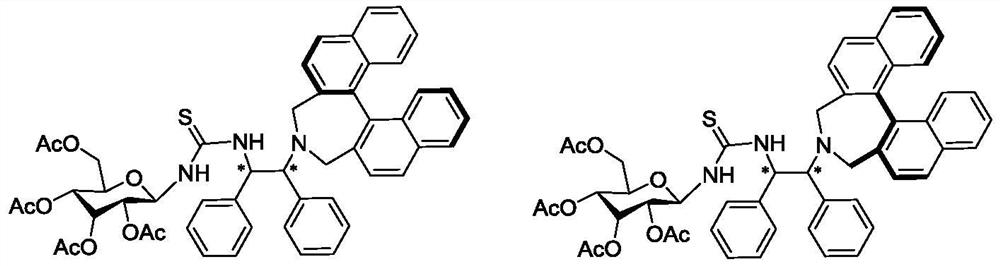
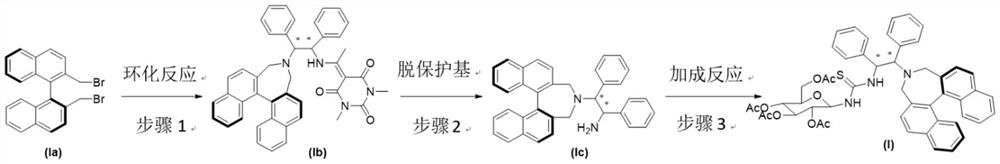
Chiral sugar-containing thiosemicarbazide catalyst derived from binaphthalene skeleton and preparation method and application thereof
The invention relates to a chiral sugar-containing thiosemicarbazide catalyst derived from binaphthalene skeleton and a preparation method and application thereof. The preparation method comprises the following steps: carrying out cyclization reaction on S or R configuration 2, 2 '-bis (bromomethyl)-1, 1'-binaphthalene (Ia) and chiral (S, S) configuration or (R, R) configuration 1, 2-diphenylethylenediamine protected by a 5-acetyl-1, 3-dimethyl barbituric acid protecting group in an organic solvent under the action of alkali catalysis to prepare an intermediate (Ib); reacting the intermediate (Ib) in an organic solvent under the action of a strong base to remove a protecting group to prepare an intermediate (Ic), reacting the intermediate (Ic) with sugar with an isothiocyanate group in the organic solvent at a low temperature, and separating and purifying to obtain the chiral sugar-containing thiosemicarbazide (I) derived from the protecting group-removed target binaphthalene skeleton. The catalyst is used for catalyzing an asymmetric decarboxylation Mannich reaction of cyclic imine, and an addition product is prepared with a high yield of 84-97% and a high enantioselectivity ee value of 83-98%.
Owner:TIANJIN UNIV
A low-melting-point nylon hot-melt yarn for preparing fly-woven shoe uppers
ActiveCN111088549BLow costLow melting pointTextile/flexible product manufactureArtifical filament manufacturePentamethylcyclopentadienePtru catalyst
The invention discloses a low-melting-point nylon hot-melt yarn for preparing fly-woven shoe uppers, which is formed by polymerizing polyamines and polyacids as reactants, and adding catalysts, modifiers and other auxiliary agents for further reaction; A mixture of decanediamine, pentaethylenehexamine and (1R,2R)‑(‑)‑N‑(p-toluenesulfonyl)‑1,2‑diphenylethylenediamine was chosen for the polyamine; A mixture of sebacic acid, terephthalic acid, and dodecenylsuccinic acid was selected; bis(pentamethylcyclopentadiene) zirconium dichloride, N,N-dimethylanilinium were selected as catalysts A mixture of tetrakis(pentafluorophenyl)borate; the modifiers are 3,5-diamino-1,2,4-triazole, bicyclo(2.2.2)oct-5-ene-2 , a mixture of 3‑dicarboxylic anhydride and 2,2‑bis[4‑(4‑aminophenoxy)phenyl]‑1,1,1,3,3,3‑hexafluoropropane. Nylon thermal fuse not only has a lower melting point, but also has greater fiber strength and elongation, and also has stronger anti-ultraviolet properties and better color fastness, and can be used to prepare fly-knit uppers.
Owner:WENZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com