Porous chiral organic polymer catalyst and preparation method thereof
A catalyst and polymer technology, applied in the field of porous chiral organic polymer catalysts and their preparation, can solve the problems of difficult catalyst recovery and industrial application, achieve excellent stability, reduce catalyst costs and subsequent product separation costs, and increase active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
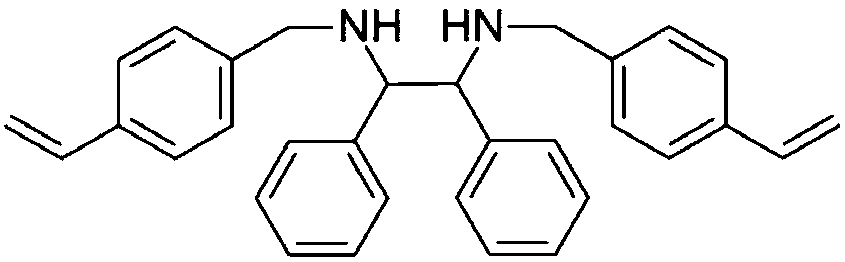
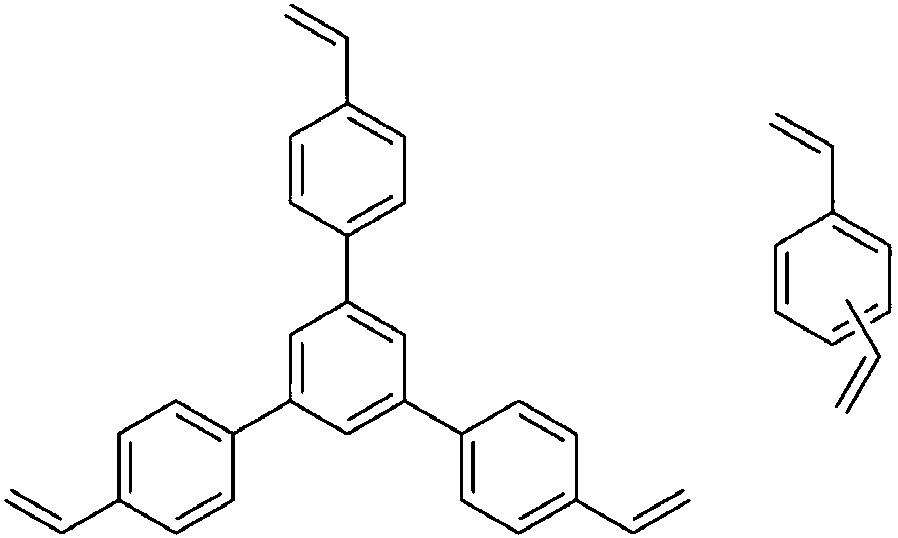
[0033] Dissolve 0.7g N,N-bis(4-vinylbenzyl)-1,2-diphenylethylenediamine in 15mL N,N-dimethylformamide solvent at 298K and inert gas Ar protection atmosphere , while adding 0.6g of 1,3,5-tri-p-styrylbenzene, and then adding 0.0325g of azobisisobutylcyanide, and stirring for 1h. The stirred solution was moved to an autoclave, and stood at 373K for 24h. After cooling to room temperature, it was washed three times with methanol solvent, and then dried in vacuum at low temperature to obtain a white powdery solid. The solid was added to a two-necked flask, 30ml of dichloromethane was added, and after the air was removed through three cycles of liquid nitrogen freezing, vacuum and melting, 43.3mg of dichloro(p-methylcumene)ruthenium(II) dichloride was added. After polymerizing, heat to 323K under stirring. After 2 hours, the solvent was removed by rotary evaporation, and a dark gray solid powder was obtained after vacuum drying, which was a porous chiral organic polymer catalyst. ...
Embodiment 2
[0035] In Example 2, 0.26 g of divinylbenzene was weighed to replace 1,3,5-tri-p-styrylbenzene, and the rest of the catalyst synthesis process was the same as in Example 1.
Embodiment 3
[0037] The catalysis prepared in Example 1 was used in the chiral hydrogenation reaction of acetophenone, and 0.0853 g of the catalyst was placed in an autoclave, and 0.24 g of acetophenone, 10 ml of isopropanol, and 0.0059 g of potassium hydroxide were added, and hydrogen was replaced in the autoclave. Gas three times, stir, heat up to 323K, and react for 5h. It has a conversion rate of 59% and an ee value of 21.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com