Cobalt complex based on chiral imidazoline as skeleton, and synthesis method and application thereof
A synthesis method and technology of cobalt complexes, applied in the directions of cobalt organic compounds, organic chemistry methods, chemical instruments and methods, etc., can solve problems such as difficult planting and unsatisfactory yield, and achieve the effect of high polymerization activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The synthetic method of the chiral imidazoline cobalt complex of the present embodiment is as follows:
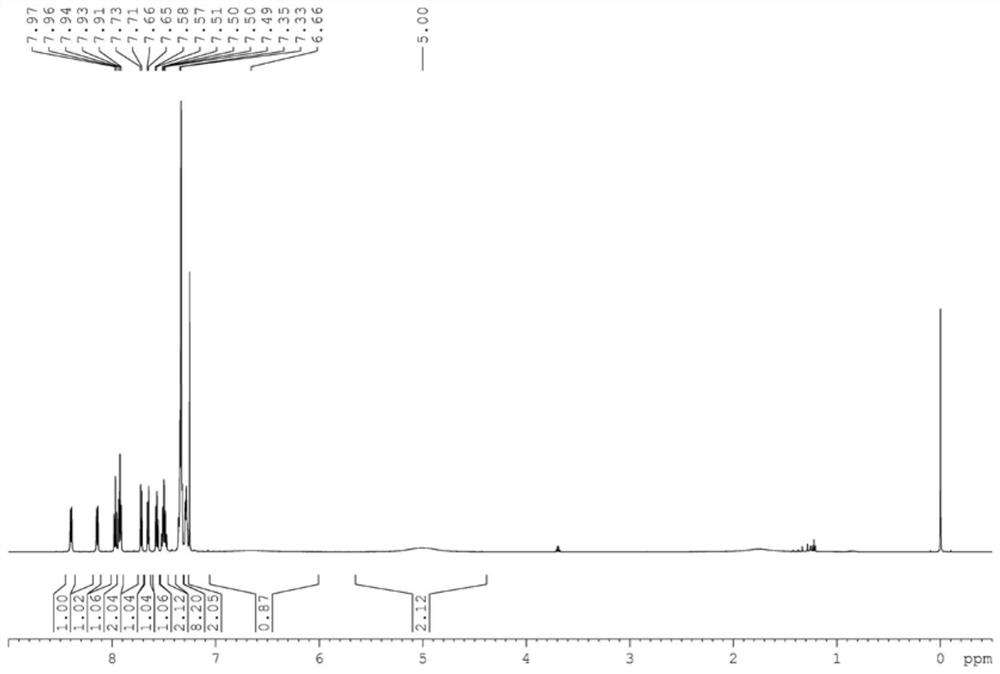
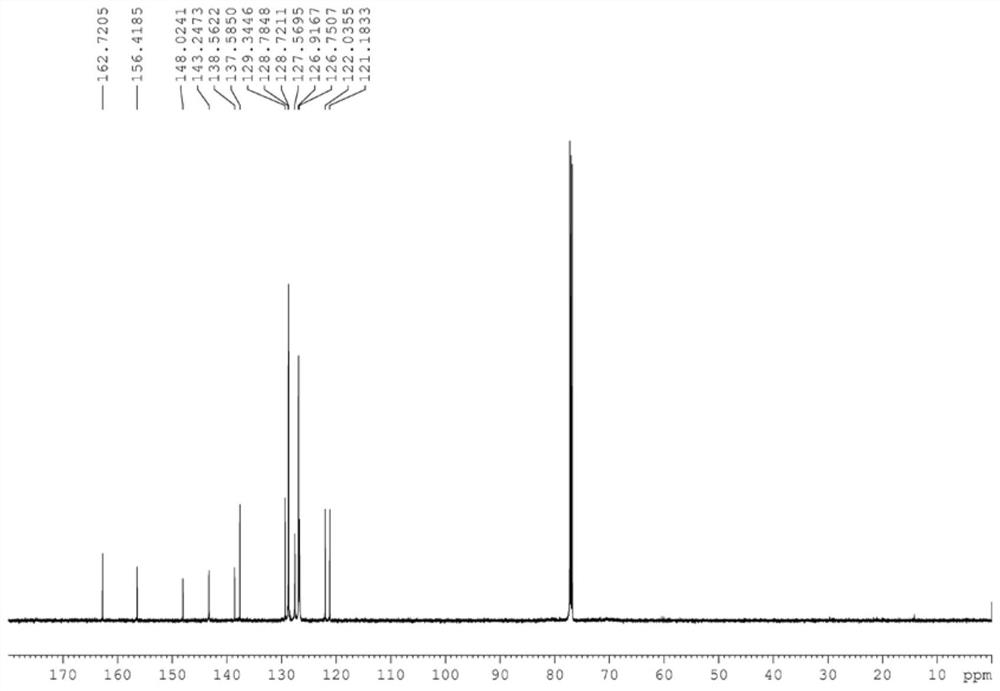
[0041] (1) Synthesis of L1: 6-naphthylpyridine-2-carbaldehyde (1.16g, 5.0mmol), (1S, 2S) diphenylethylenediamine (1.16g, 5.5mmol), iodine element (1.46g, 5.75mmol), potassium carbonate (2.07g, 15mmol) were added in the 250mL round bottom flask, then 80mL of tert-butanol was added, and reacted at 70°C for 3 hours. After the reaction was completed, the saturated solution of sodium thiosulfate was added to quench, Cool to room temperature, extract with ethyl acetate, take the organic phase, spin to dry the solvent, and separate by column chromatography (petroleum ether / ethyl acetate=6 / 1) to obtain the target complex.
[0042] (2) Synthesis of Co 1: Add the above ligand compound (149.6mg, 0.5mmol) and cobalt chloride (64.9mg, 0.5mmol) into a 25mL Shrek bottle, then add 10mL of anhydrous tetrahydrofuran, at room temperature The reaction was stirred under argon atmosphere...
Embodiment 2
[0044] The synthetic method of the chiral imidazoline cobalt complex of the present embodiment is as follows:
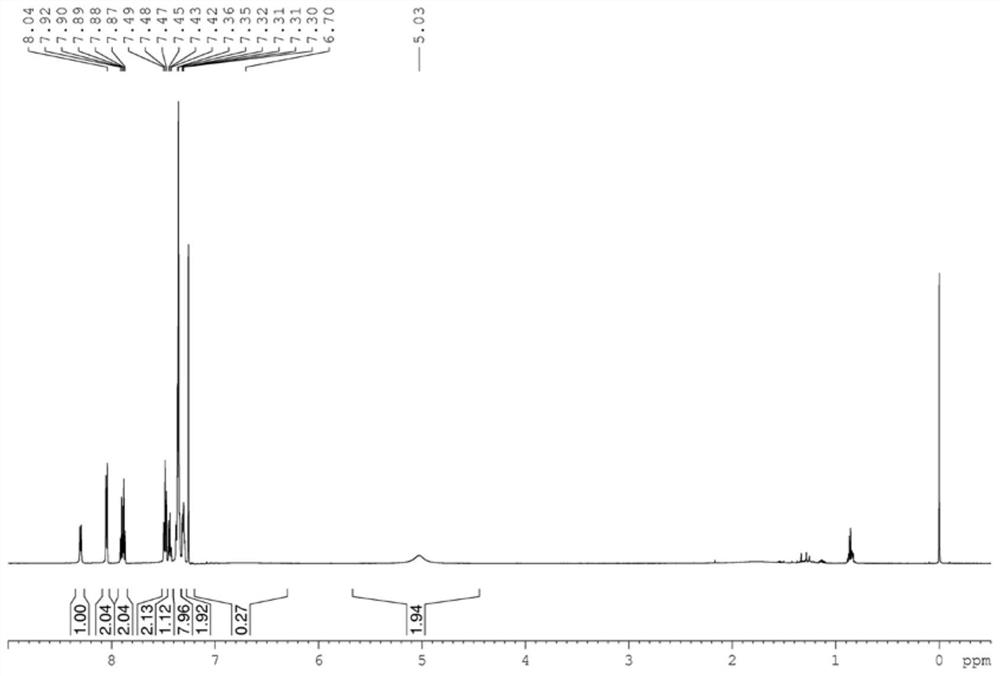
[0045] (1) Synthesis of L2: 6-phenylpyridine-2-carbaldehyde (916.1mg, 5.0mmol), (1S,2S)diphenylethylenediamine (1.16g, 5.5mmol), iodine (1.46g, 5.75mmol), potassium carbonate (2.07g, 15mmol) were added in the 250mL round bottom flask, then 80mL of tert-butanol was added, and reacted at 70°C for 3 hours. After the reaction was completed, the saturated solution of sodium thiosulfate was added to quench, Cool to room temperature, extract with ethyl acetate, take the organic phase, spin to dry the solvent, and separate by column chromatography (petroleum ether / ethyl acetate=20 / 1) to obtain the target complex.
[0046] (2) Synthesis of Co 2: Add the above-mentioned ligand compound (252.6mg, 0.5mmol) and cobalt chloride (64.9mg, 0.5mmol) in a 25mL Shrek bottle, then add 10mL of anhydrous tetrahydrofuran, at room temperature The reaction was stirred under argon atmosphere ...
Embodiment 3
[0048] The synthetic method of the chiral imidazoline cobalt complex of the present embodiment is as follows:
[0049] (1) Synthesis of L3: 2-pyridinecarbaldehyde (535.6mg, 5.0mmol), (1S, 2S) diphenylethylenediamine (1.16g, 5.5mmol), iodine (1.46g, 5.75mmol), carbonic acid Potassium (2.07g, 15mmol) was added in a 250ml round-bottomed flask, then 80mL of tert-butanol was added, and reacted at 70°C for 3 hours. After the reaction was completed, a saturated solution of sodium thiosulfate was added to quench it, cooled to room temperature, and used Extract with ethyl acetate, take the organic phase, spin to dry the solvent, and separate by column chromatography (petroleum ether / ethyl acetate=6 / 1) to obtain the target complex.
[0050] (2) Synthesis of Co 3: Add the above ligand complex (149.6mg, 0.5mmol) and cobalt chloride (64.9mg, 0.5mmol) into a 25mL Shrek bottle, then add 10mL of anhydrous tetrahydrofuran, at room temperature conditions, the reaction was stirred under argon f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com