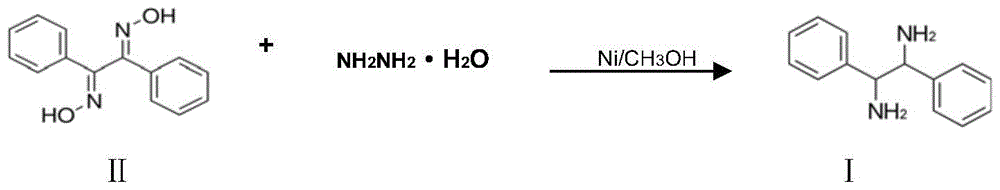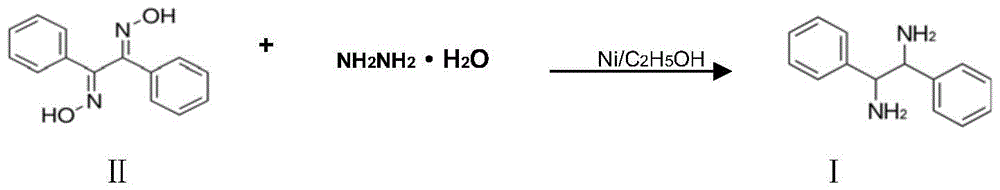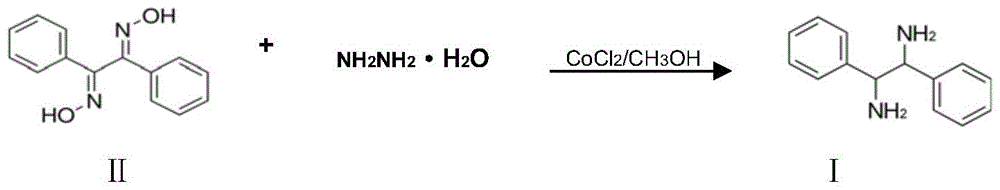A kind of preparation method of (±)‑1,2‑diphenylethylenediamine
A technology for diphenylethylenediamine and diphenylethylenedione dioxime, which is applied in the field of preparation of -1,2-diphenylethylenediamine, can solve problems such as difficulties in industrialization, and achieves environmental friendliness and ease of use. Industrial production conditions and the effect of less by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Add 1,2-diphenylethanedionedioxime (48g, 0.20mol) and 260ml of methanol into a 500ml four-necked flask equipped with a condenser, stir to dissolve them all, then add 2g of activated carbon (120 mesh), 0.5 g Raney nickel, heat up to 60°C, slowly add 80% hydrazine hydrate (28.75g, 0.46mol) dropwise, keep the reaction temperature between 58°C-62°C, nitrogen will be released during the reaction, and the dropping rate should be controlled under nitrogen The release rate should not be too fast, and the liquid chromatography should track the reaction process. When the raw material 1,2-diphenylethylenedionedioxime disappears, the reaction is completed, the mixed solution is cooled, Raney nickel and activated carbon are filtered, and a small amount of water is covered for storage. For further use, the filtrate was distilled to recover methanol, the residue was added to 200ml of petroleum ether and heated to dissolve it, cooled for 24 hours and filtered to obtain 41.55g of (±)-1,2...
Embodiment 2
[0036] Add 1,2-diphenylethanedione dioxime (48g, 0.20mol) and 300ml ethanol into a 500ml four-necked flask equipped with a condenser, stir to dissolve them all, then add 2g of activated carbon (120 mesh), 0.5 g Raney nickel, heat up to 60°C, slowly add 80% hydrazine hydrate (28.75g, 0.46mol) dropwise, keep the reaction temperature between 58°C-62°C, nitrogen will be released during the reaction, and the dropping rate should be controlled under nitrogen The release rate should not be too fast, and the liquid chromatography should track the reaction process. When the raw material 1,2-diphenylethylenedionedioxime disappears, the reaction is completed, the mixed solution is cooled, Raney nickel and activated carbon are filtered, and a small amount of water is covered for storage. For further use, the filtrate was distilled to recover ethanol, the residue was added to 200ml of petroleum ether and heated to dissolve it, cooled for 24 hours and filtered to obtain 41.13g of (±)-1,2-dip...
Embodiment 3
[0040] Add 1,2-diphenylethanedione dioxime (48g, 0.20mol) and 300ml methanol into a 500ml four-necked flask equipped with a condenser, stir to make it all dissolve, then add 2g of activated carbon (120 mesh), 1.9 g cobalt chloride, heat up to 60°C, slowly add 50% hydrazine hydrate (46g, 0.46mol) dropwise, keep the reaction temperature between 60°C-62°C, nitrogen will be released during the reaction, and the dropping rate will be controlled at the nitrogen release The speed should not be too fast, and the liquid chromatography should track the reaction process. When the raw material 1,2-diphenylethylenedionedioxime disappears, the reaction is completed, the mixed solution is cooled, Raney nickel and activated carbon are filtered, and a small amount of water is covered to preserve it. For further use, the filtrate was distilled to recover methanol, the residue was added to 200ml of petroleum ether and heated to dissolve it, cooled for 24 hours and filtered to obtain 36.46g of (±)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com