Chiral sugar-containing thiosemicarbazide catalyst derived from binaphthalene skeleton and preparation method and application thereof
A technology of thiosemicarbazide and binaphthalene is applied in the preparation of sugar derivatives, chemical instruments and methods, catalysts for physical/chemical processes, etc., and can solve the problems of sometimes poor stereo control and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
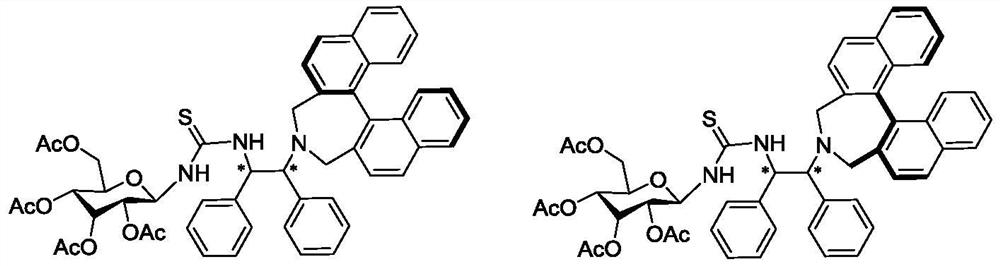
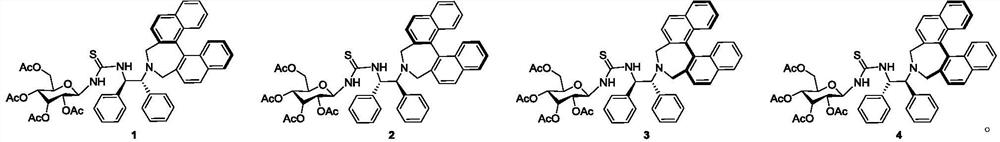
[0022] Example 1: (D.S.R.R.-binaphthalene-1,2-diphenylethylenediaminetetraacetylglucothiourea) (Catalyst 1)
[0023]
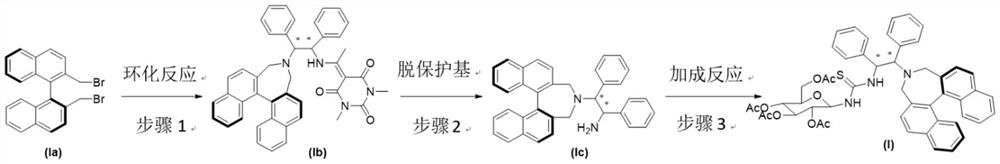
[0024] Step 1: 2,2'-bis(bromomethyl)-1,1'-binaphthyl Ia (969mg, 2.2mmol) containing S configuration, 5-acetyl-1,3-dimethylbaby N,N - Dimethylformamide (12 mL) solution was stirred at 45° C. for 72 hours under argon protection. The reaction was quenched with distilled water (60 mL). The aqueous phase was extracted four times with ethyl acetate (120 mL), the organic phase was washed once with 50 mL of saturated brine, dried by adding anhydrous sodium sulfate, the solvent was removed under reduced pressure, and separated by a neutral alumina column (petroleum ether / ethyl acetate = 6 / 1 as the eluent) to obtain light yellow solid intermediate Ib (1.19g, yield 89%). 1 H NMR (400MHz, CDCl 3 )δ2.44(s,3H),3.27(d,J=12.3Hz,2H),3.31(s,3H),3.45(s,3H),3.90(d,J=12.3Hz,2H),4.16( d,J=5.1Hz,1H),5.30(dd,J=5.1,7.5Hz,1H),7.16-7.43(m,18H),7.82(d,J=8.2Hz,2H),7.89(d,J =8.2Hz...
Embodiment 2
[0027] Example 2: (D.S.S.S-binaphthalene-1,2-diphenylethylenediaminetetraacetylglucothiourea) (catalyst 2)
[0028]
[0029] Step 1: 2,2'-bis(bromomethyl)-1,1'-binaphthyl Ia (969mg, 2.2mmol) containing S configuration, 5-acetyl-1,3-dimethylbaby Diphenylethylenediamine (786mg, 2mmol) and triethylamine (610μl, 4.4mmol) in tetrahydrofuran (12mL) in (1S, 2S) configuration protected by the acid protecting group (DAB) were placed under argon protection in Stir at 50°C for 48 hours. The reaction was quenched with distilled water (60 mL). The aqueous phase was extracted four times with ethyl acetate (120 mL), the organic phase was washed once with 50 mL of saturated brine, dried by adding anhydrous sodium sulfate, the solvent was removed under reduced pressure, and separated by a neutral alumina column (petroleum ether / ethyl acetate = 6 / 1 as the eluent) to obtain light yellow solid intermediate Ib (1.13g, yield 85%). 1 H NMR (400MHz, CDCl 3 )δ2.43(s,3H),3.27(d,J=12.4Hz,2H),3.31...
Embodiment 3
[0032] Example 3: (D.R.R.R-binaphthalene-1,2-diphenylethylenediaminetetraacetylglucothiourea) (catalyst 3)
[0033]
[0034] Step 1: 2,2'-bis(bromomethyl)-1,1'-binaphthyl Ia (969 mg, 2.2 mmol) containing (R) configuration, 5-acetyl-1,3-dimethyl A solution of diphenylethylenediamine (786mg, 2mmol) and DABCO (492mg, 4.4mmol) in (1R, 2R) configuration protected by a barbituric acid protecting group (DAB) in dichloromethane (12mL) was protected under argon. Stir at 50°C for 48 hours. The reaction was quenched with distilled water (60 mL). The aqueous phase was extracted four times with ethyl acetate (120 mL), the organic phase was washed once with 50 mL of saturated brine, dried by adding anhydrous sodium sulfate, the solvent was removed under reduced pressure, and separated by a neutral alumina column (petroleum ether / ethyl acetate = 6 / 1 as the eluent) to obtain light yellow solid intermediate Ib (1.02g, yield 75%). 1 H NMR (400MHz, CDCl 3 )δ2.41(s,3H),3.27(d,J=12.2Hz,2H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com