Chiral primary amine malonamide compound as well as preparation method and application thereof
A primary amine malonamide and compound technology, applied in the field of chiral primary amine malonamide compounds and their preparation, can solve the problems of low field control effect, few practical varieties, environmental risks and the like, and achieve good resistance to tobacco flowers. Effect of Leaf Virus Activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
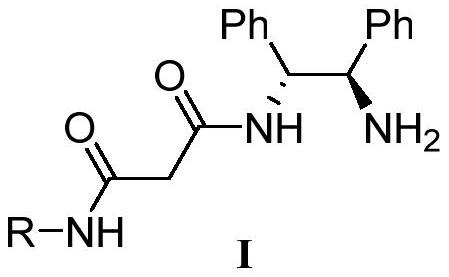
[0025] N shown in chemical structural formula I-1 1 -((1R,2R)-2-amino-1,2-diphenylethyl)-N 3 -The preparation method of phenylmalonamide is as reaction formula one:
[0026]
[0027] N 1 -((1R,2R)-2-amino-1,2-diphenylethyl)-N 3 -The chemical structural formula I-1 of phenylmalonamide is
[0028]
[0029] The concrete steps of its preparation method are as follows:
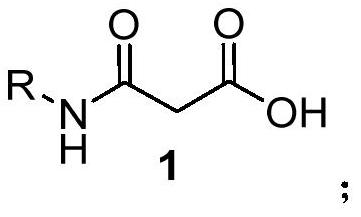
[0030] In the first step, 3-oxo-3-(phenylamino)propionic acid (1.79g, 10mmol), (1R,2R)-N-tert-butoxycarbonyl-1,2-diphenylethylenediamine ( 3.12g, 10mmol), 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (2.30g, 12mmol), diisopropylethylamine (DIPEA, 2.58g, 20mmol) 1-Hydroxybenzotriazole (HOBt, 1.62g, 12mmol) was added to 50mL THF, stirred at room temperature at 25°C for 8 hours, after the reaction was completed, the solvent was removed in vacuo, and the residual liquid was dissolved in 30mL dichloromethane and saturated saline After washing, the organic phase was dried to obtain the crude pro...
Embodiment 2
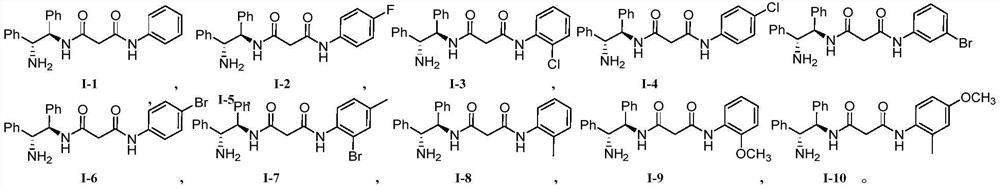
[0033] N shown in chemical structural formula I-2 1 -((1R,2R)-2-amino-1,2-diphenylethyl)-N 3 The preparation method of -(4-fluorophenyl) malonamide is as follows:
[0034] N 1 -((1R,2R)-2-amino-1,2-diphenylethyl)-N 3 The chemical structural formula I-2 of -(4-fluorophenyl) malonamide is
[0035]
[0036] The concrete steps of its preparation method are as follows:
[0037] In the first step, except that 3-((4-fluorophenyl)amino)-3-oxopropionic acid is used to replace 3-oxo-3-(phenylamino)propionic acid, other experimental steps are the same as in Example 1, Go to the next step directly without purification;
[0038] In the second step, the crude product obtained in the previous step is processed according to the experimental procedure of Example 1 to obtain a light yellow solid with a yield of 96%; after determination, the relevant parameters of the light yellow solid are: 1 H NMR (400MHz, CDCl 3 )δ9.47(s,1H),8.05(s,1H),7.42(dd,J=8.8,4.9Hz,2H),7.29(m,7.9Hz,11H),7.18(...
Embodiment 3
[0040] N shown in chemical structural formula I-3 1 -((1R,2R)-2-amino-1,2-diphenylethyl)-N 3 The preparation method of -(2-chlorophenyl) malonamide is as follows:
[0041] N 1 -((1R,2R)-2-amino-1,2-diphenylethyl)-N 3 The chemical structural formula I-3 of -(2-chlorophenyl) malonamide is
[0042]
[0043] The concrete steps of its preparation method are as follows:
[0044] In the first step, except that 3-((2-chlorophenyl)amino)-3-oxopropionic acid is used to replace 3-oxo-3-(phenylamino)propionic acid, other experimental steps are the same as in Example 1, Go to the next step directly without purification;
[0045] In the second step, the crude product obtained in the previous step was processed according to the experimental procedure of Example 1 to obtain a light yellow solid with a yield of 87%; after determination, the relevant parameters of the light yellow solid were: 1 H NMR (400MHz, CDCl 3 )δ9.48(s,1H),8.33(dd,J=8.2,1.5Hz,1H),7.54(d,J=8.3Hz,1H),7.42–7.30(m,8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com