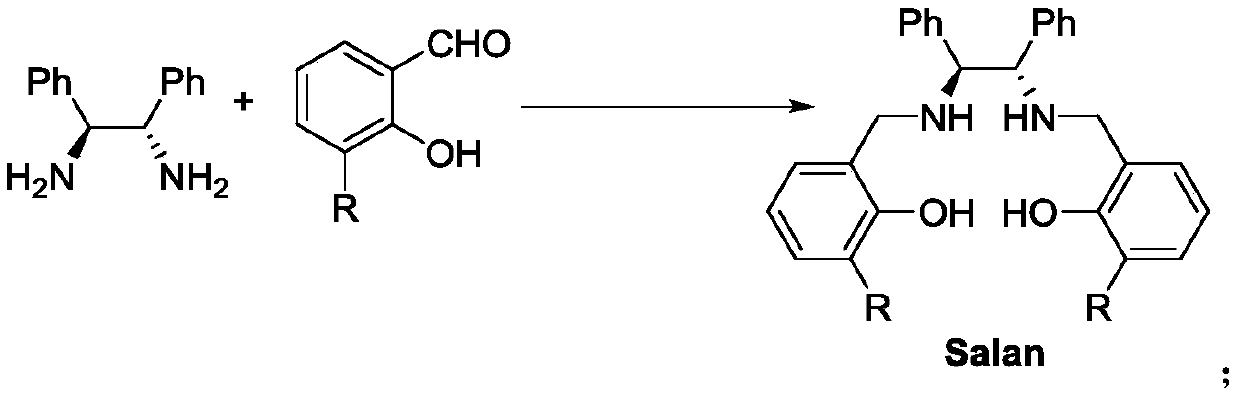
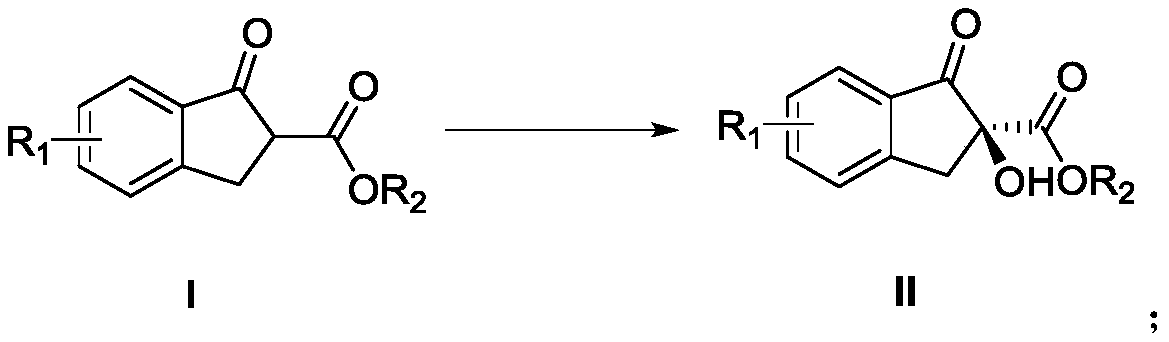
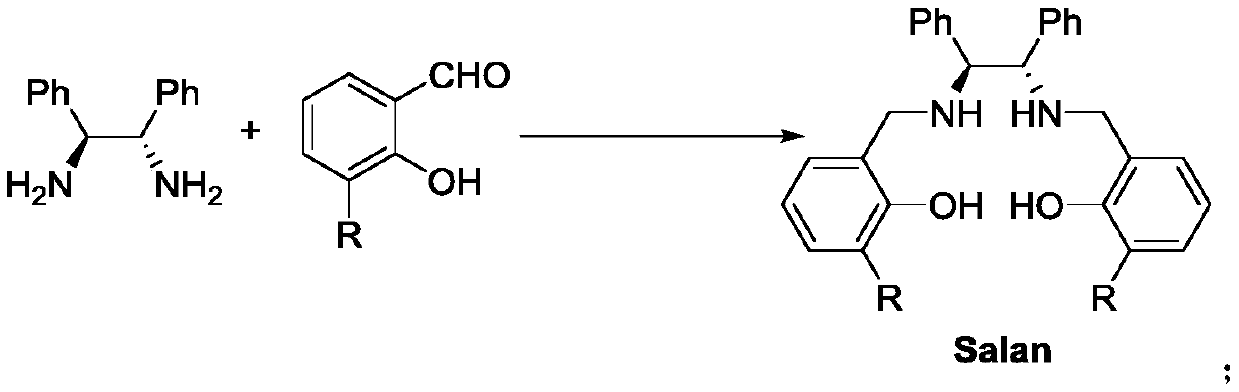
Salan ligand, metal-Salan complex and preparation method of chiral alpha-hydroxy-beta-keto ester compound
A technology of keto esters and complexes, which is applied in the field of organic synthesis catalysis, can solve problems such as not optimistic, and achieve the effects of high enantiomeric excess value, good application prospects, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Preparation of ligand L3 (R is 2-methoxyphenyl)
[0028]
[0029] Under argon protection, dissolve (1S,2S)-1,2-diphenylethylenediamine (1.0mmol, 212mg, 1.0equiv) in 50mL of absolute ethanol, and then add 3-(2-methoxy)benzene Base salicylaldehyde (2.0mmol, 456mg, 2.0equiv), continue to stir for 10min, then add 5 drops of acetic acid successively, Molecular sieve. The reaction solution was refluxed overnight. Cool down to 0°C, add NaBH in batches 4 (3.0 mmol, 113 mg, 3.0 equiv), stirred overnight at room temperature. After the reaction was completed, the resulting mixed solution was evaporated to dryness, and then the resulting solid was added with NH 4 Cl quenched, stirred for 30min, CH 2 Cl 2 Extract 3 times. Wash twice with saturated saline, anhydrous NaSO 4 Dry, evaporate the solvent to dryness, and recrystallize from ethanol to obtain L3 (541 mg, 0.85 mmol, 85% yield).
[0030] (c=0.5, CHCl 3 );
[0031] 1 H NMR (600MHz, CDCl 3 )δ9.73(s,2H),7.33(td...
Embodiment 2
[0036] Optimization of reaction conditions
[0037]
[0038] Dissolve metal compound (5.0mol%) and ligand (5.5mol%) in 2mL organic solvent, stir at room temperature for 30min, then add methyl 5-chloroindanone formate (1.0equiv.) and oxidizing agent (1.5equiv.) successively, Continue to react for 4h. After the reaction is complete, use CH 2 Cl 2 Extracted 3 times, and the organic phase was washed 2 times with saturated brine. with anhydrous Na 2 SO 4 After drying, filtering, and evaporating the resulting solution to dryness, a crude product was obtained, which was separated by column chromatography to obtain a pure product.
[0039] Table 1 condition optimization
[0040]
[0041]
[0042] a 0°C; b 50°C; c 30%H 2 o 2 ; d tert-butyl hydroperoxide (TBHP);e Add additive acetic acid; f HPLC: OD-H (5μm, 250 mm×4.6mm), 25℃, hexane / i-PrOH=90 / 10, 1mL / min, 254nm, t R1 (major)=12.5min,t R2 (minor) = 15.7 min.
[0043] We know from Table 1 that the corresponding...
Embodiment 3
[0045] Preparation of Chiral α-Hydroxy-β-Ketoesters
[0046]
[0047] The above formula shows the preparation process of α-hydroxy-β-ketoester: Dissolve L3 (5.5mol%, 3.5mg, 0.055equiv.) in 2 mL toluene, add Zr(acac) 4 (5.0mol%, 2.4mg, 0.05equiv.), stirred at room temperature for 30min to prepare the Zr(IV)-Salan complex, and then added I (0.1mmol, 1.0equiv.), cumene hydroperoxide (CHP; 80 %wt, 0.15mmol, 28.5 mg, 1.5equiv.), and continued stirring at room temperature for 4h. After the reaction was completed, α-hydroxy-β-ketoester II was separated by column chromatography (petroleum ether / ethyl acetate=8 / 1). The product yield, ee value and detection method thereof are shown in Table 2.
[0048] Table 2 α-Hydroxy-β-keto ester data analysis
[0049]
[0050]
[0051]
[0052] From Table 2, we found that the yield and ee value of the obtained α-hydroxy-β-ketoester were between 92–99% and 90–99%, respectively, without considering the influence of electron-withdrawing ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com