Preparation method of chiral diphenylethylenediamine ruthenium complex
A technology of diphenylethylenediamine ruthenium and complex compounds, which is applied in the directions of ruthenium organic compounds, organic chemical methods, chemical instruments and methods, etc., can solve the problem of increased metal consumption, reduced product yield, low product purity, etc. problems, to achieve the effect of improving metal utilization, reducing production costs, and simple operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
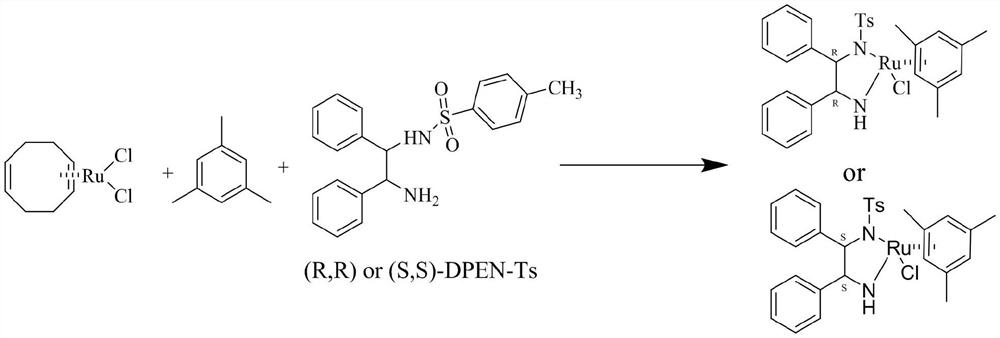
[0022] The invention provides a kind of preparation method of chiral diphenylethylenediamine ruthenium complex, comprising the following steps:
[0023] (a) react after mixing (1,5-cyclooctadiene) ruthenium dichloride, an organic solvent and 1,3,5-trimethylbenzene to obtain a solution system A;
[0024] (b) mixing [(1R,2R) or (1S,2S)-N-(2-amino-1,2-diphenylethyl)]p-toluenesulfonamide with an organic solvent to obtain a solution system B;
[0025] (c) react after mixing the solution system A, the solution system B and the alkali reagent, after the reaction is completed, sequentially cool, filter, wash, drain, and vacuum-dry to obtain the chiral diphenylethylenediamine ruthenium complex;
[0026] The chiral diphenylethylenediamine ruthenium complex is {[(1R, 2R) or (1S, 2S)-(-)-2-amino-1,2-diphenylethyl] (p-toluene Sulfonyl)amino}(mesitylene)ruthenium chloride.
[0027] In the present invention, the reactions in the steps (a), (b) and (c) are independently carried out in an ox...
Embodiment 1
[0043] (a) Mix 14.01g (1,5-cyclooctadiene) ruthenium dichloride and 60mL absolute ethanol at 65°C in an oxygen-free atmosphere, then add 24.04g of 1,3,5-tri Toluene was stirred and reacted for 5h to obtain solution system A;
[0044] (b) Mix 19.30g of the ligand (1R,2R)-N-(2-amino-1,2-diphenylethyl)p-toluenesulfonamide and 60mL of petroleum ether at room temperature under oxygen-free conditions, and stir until clarified to obtain solution system B;
[0045] (c) Under stirring and anaerobic state, add 20.51 g of anhydrous sodium acetate to the solution system A obtained in step (a), stir for 5 minutes to obtain a mixed solution, and then add solution system B obtained in step (b) dropwise Add it to the mixture, add it dropwise for 10 minutes, and continue to stir and react for 3 hours at 65°C. After the reaction is complete, cool, filter, wash, and dry it, and dry it in vacuum at 60°C for 5 hours to obtain 28.40 g of the target product. The yield is 91.3%, and the product pur...
Embodiment 2
[0048] (a) Mix 14.01g (1,5-cyclooctadiene) ruthenium dichloride and 90mL isopropanol at 75°C in an oxygen-free atmosphere, then add 30.14g of 1,3,5-tri Toluene was stirred and reacted for 4h to obtain solution system A;
[0049] (b) At room temperature and under anaerobic conditions, mix 20.14g of ligand (1S,2S)-N-(2-amino-1,2-diphenylethyl)p-toluenesulfonamide and 150mL of cyclohexane, and stir To clarification, obtain solution system B;
[0050] (c) Under stirring and anaerobic state, add 26.51 g of anhydrous sodium acetate to the solution system A obtained in step (a), stir for 15 minutes to obtain a mixed solution, and then add the solution system B obtained in step (b) dropwise Add to the mixture, add dropwise for 15 minutes, and continue to stir and react for 2.5 hours at 75°C. After the reaction is complete, cool, filter, wash, dry, and vacuum dry at 70°C for 4 hours to obtain 28.19g of the target product , yield 90.6%, product purity 98.7%.
[0051] The elemental an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com