Chiral tridentate imine P, N, N-ligand, preparation method and application of chiral tridentate imine P, N, N-ligand in Cu-catalyzed asymmetric propargyl conversion
A technology of tridentate imine and chirality, which is applied in the field of novel chiral tridentate imine P,N,N-ligands and its preparation, can solve the problems of limited chiral ligands, and achieve easy modification of structure and properties The effect of stability and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
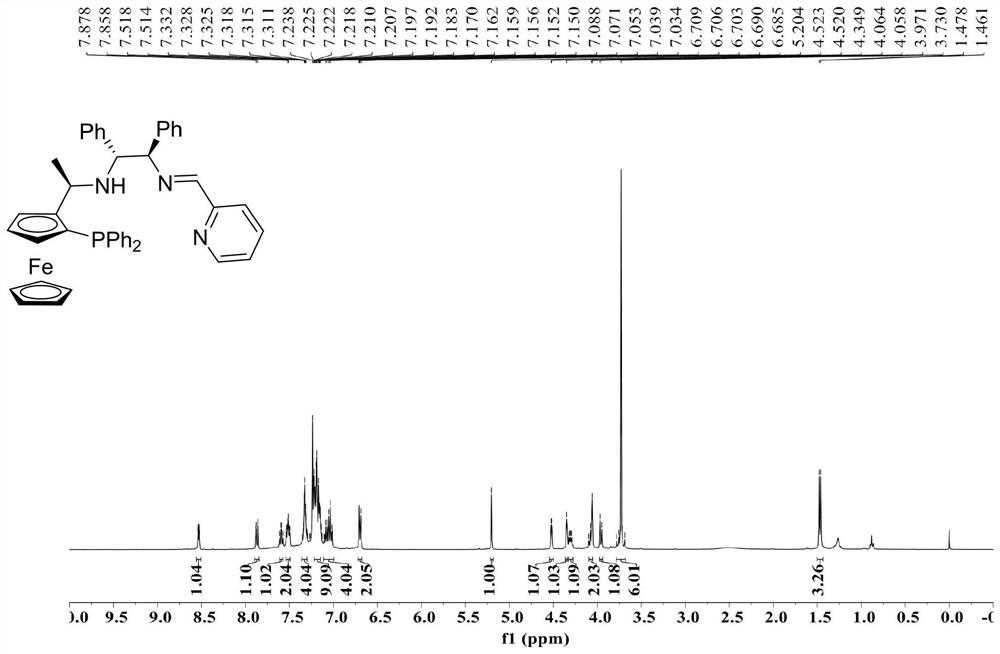
[0030] Example 1 Consisting of chiral ferrocene-1,2-diphenylethylenediamine compound (R c ,S p ,S c ,S c-II-1 and 2-pyridinecarboxaldehyde III-1 are raw materials for the preparation of chiral tridential P, N, N-imine ligands (R c ,S p ,S c ,S c )-I-1。
[0031]
[0032] Under nitrogen protection, chiral ferrocene-1,2-diphenylethylenediamine compound (R. 2-diphenylethylenediamine compound) was added to the reaction bottle c ,S p ,S c ,S c -II-1 (1.0mmol, 1.0equiv), 2-pyridinecarboxaldehyde III-1 (1.0mmol, 1.0equiv) and Anhydrous Na 2 SO 4 (2.0mmol, 2.0equiv), add 5.0mL of anhydrous toluene, reflux stirring reaction for 24h. After the reaction, the silica gel column is separated by chromatography, the vacuum is dried to obtain a yellow solid, and the yield is 81%.
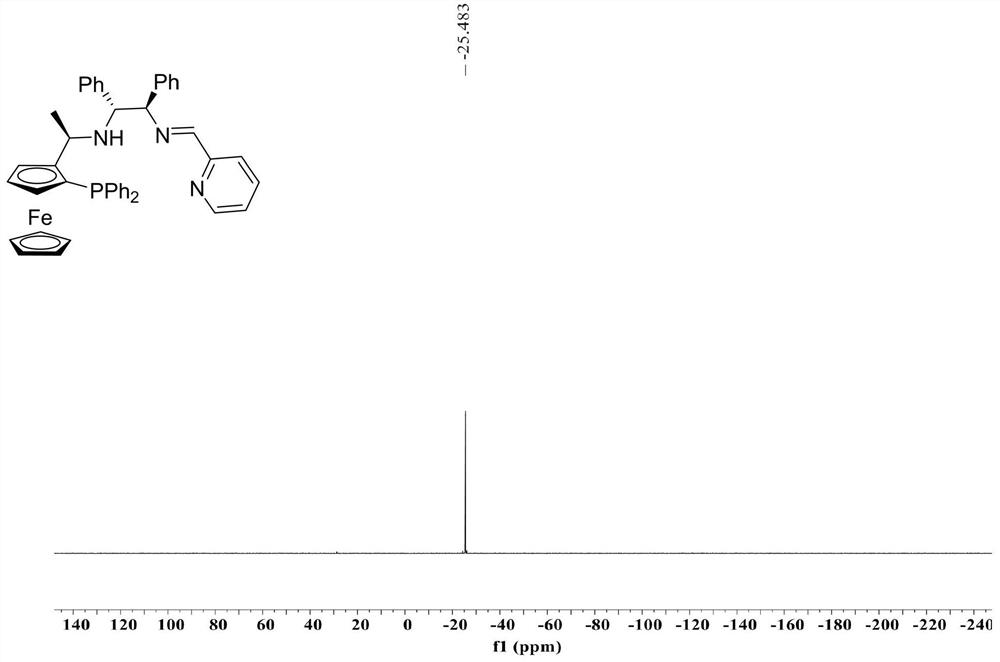
[0033] Chiral tridenta P, N, N-imine ligand (R c ,S p ,S c ,S c Nuclear magnetic resonance hydrogen spectroscopy and phosphorus spectroscopy of I-1 such as Figure 1 、 Figure 2 As shown: 1 H NMR(400MHz,CDCl 3 )δ8.54–6....
Embodiment 2
[0034] Example 2 Ethanol prepared as a reaction solvent (R c,S p ,S c ,S c )-I-1
[0035] The solvent toluene in Example 1 is replaced with ethanol, the rest is the same as Example 1. The reaction yields (R c ,S p ,S c ,S c -I-1,74% yield.
Embodiment 3
[0036] Example 3 Methanol prepared as a reaction solvent (R c ,S p ,S c ,S c )-I-1
[0037] The solvent toluene in Example 1 is replaced with methanol, the rest is the same as Example 1. The reaction yields (R c ,S p ,S c ,S c -I-1, 42% yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com