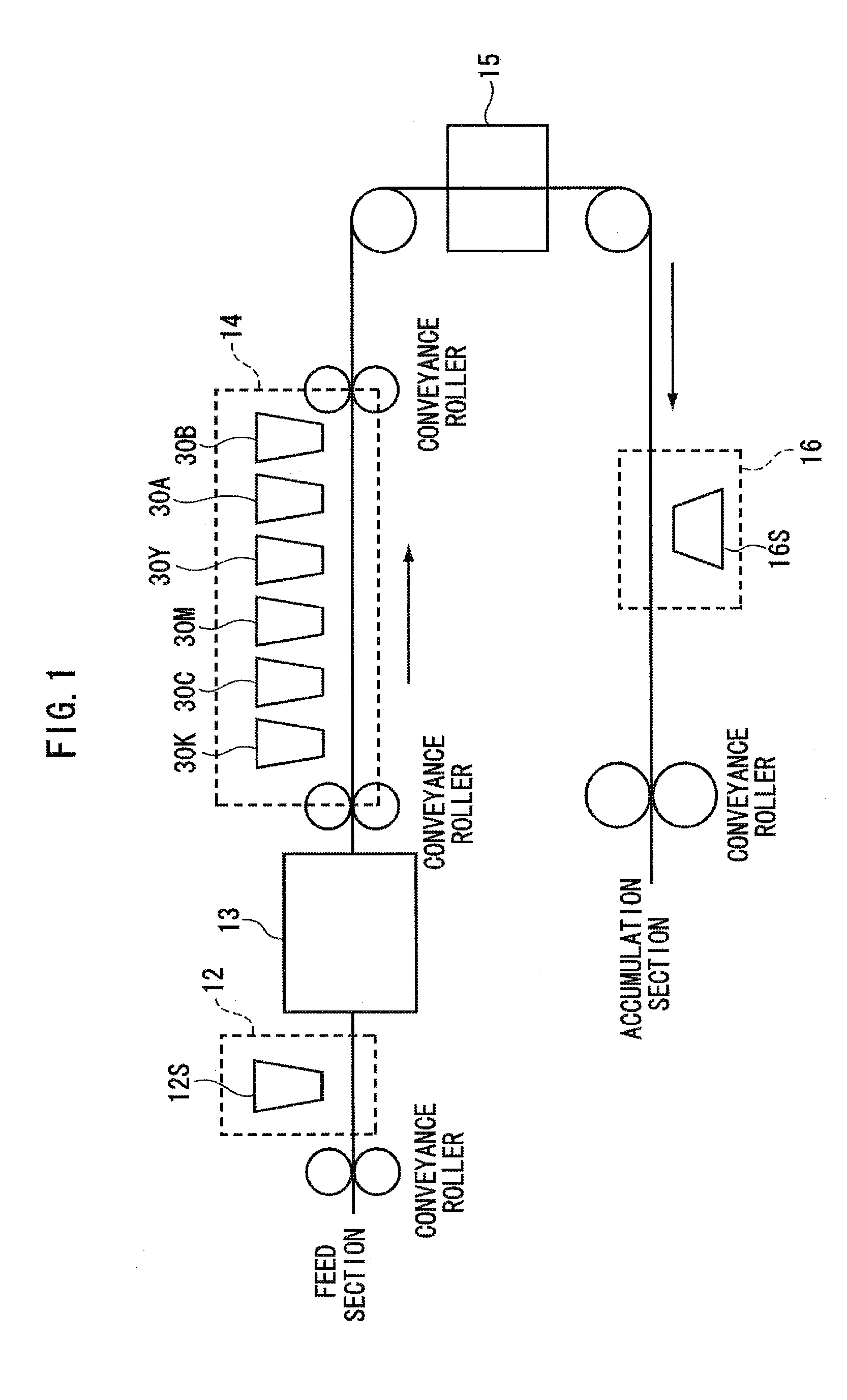
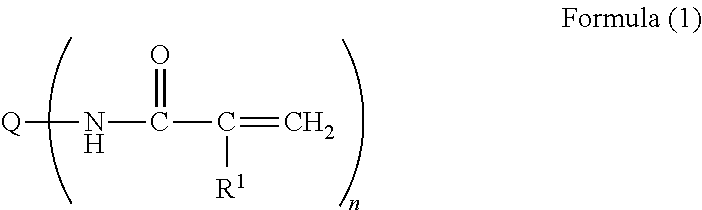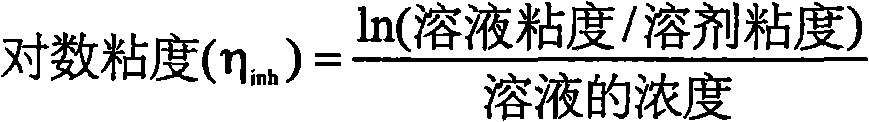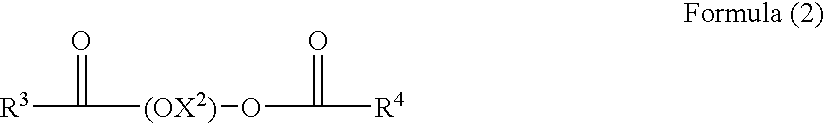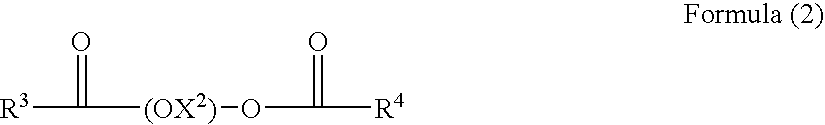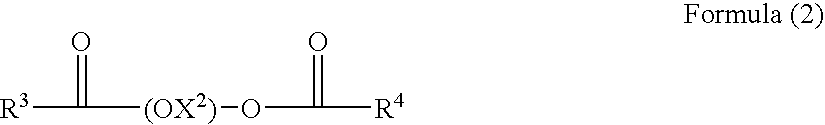Patents
Literature
107 results about "2-imidazolidinone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
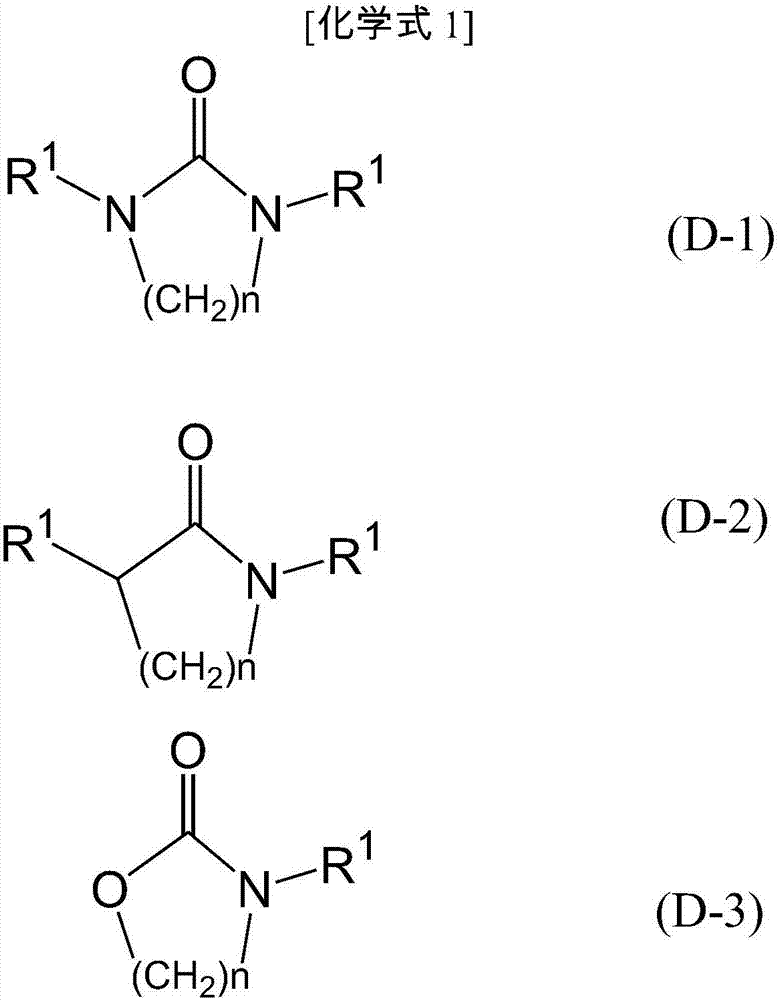
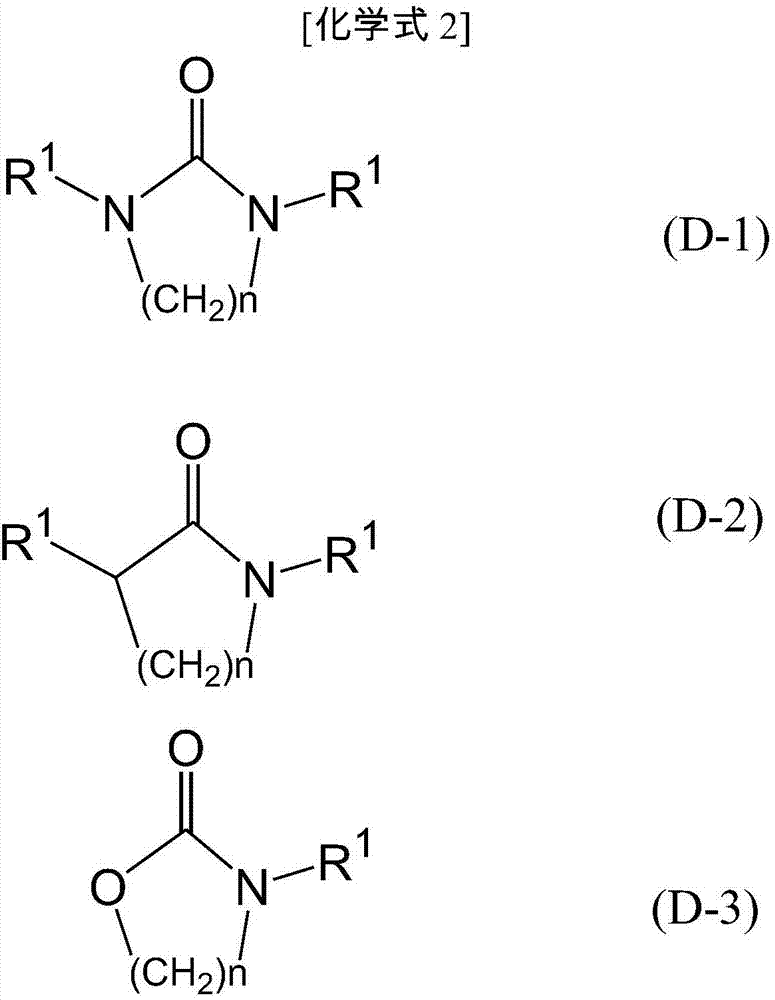
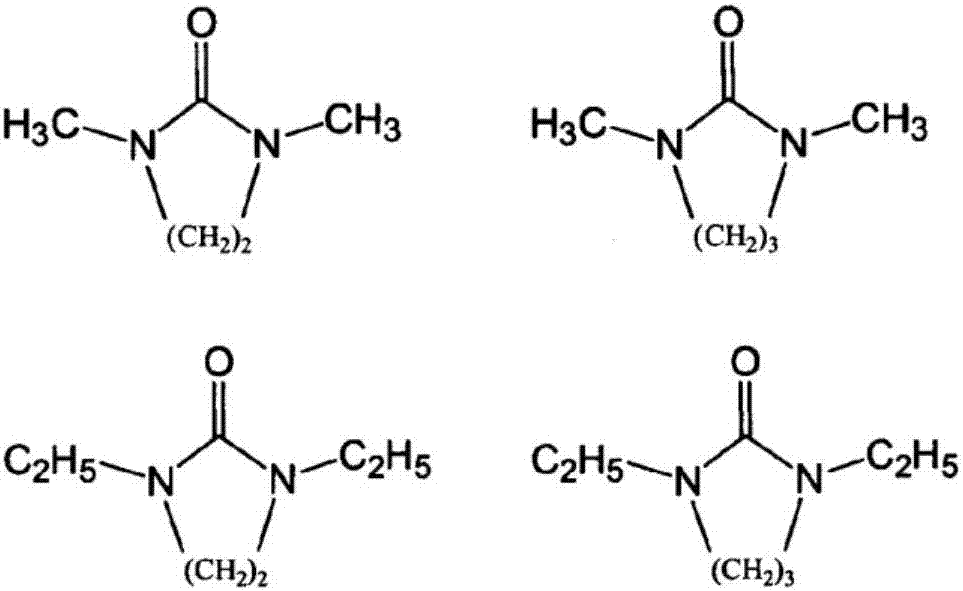
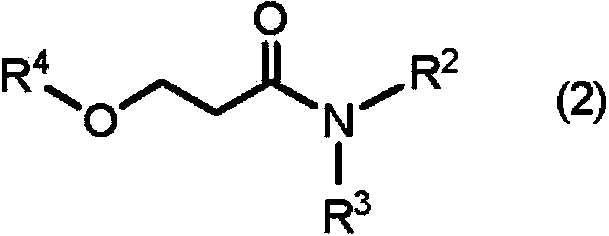
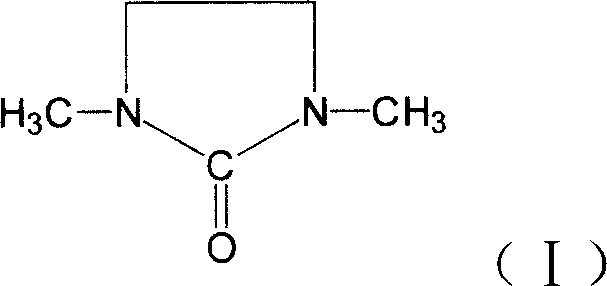
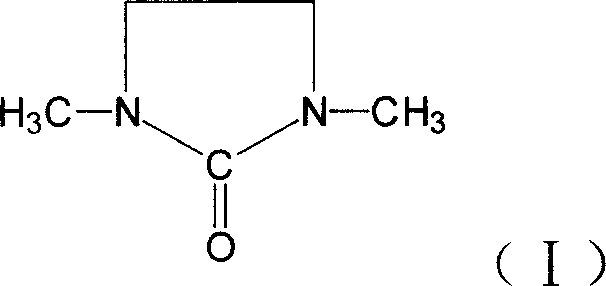
1,3-Dimethyl-2-imidazolidinone (DMI) is a cyclic urea used as a high-boiling polar aprotic solvent. It is colourless, highly polar solvent has high thermal and chemical stability. It is a homolog of the related solvent DMPU .
Polishing composition
InactiveUS20090289217A1Satisfactory polishing rateEasy to manufactureOther chemical processesSemiconductor/solid-state device manufacturingEthylenediamineCarboxylic acid
A polishing composition which achieves surfaces with high planarity and the reduction of corrosions in the wiring metal surface at the same time is provided.Such compositions include(A) an oxidizing agent;(B) at least one acid selected from an amino acid, a carboxylic acid of no more than 8 carbon atoms, and an inorganic acid;(C) a sulfonic acid having a concentration of 0.01% by mass or more and having an alkyl group of 8 or more carbon atoms;(D) a fatty acid having a concentration of 0.001% by mass or more and having an alkyl group of 8 or more carbon atoms; and(E) at least one compound selected from a pyridine carbonyl compound, a nonionic water-soluble polymer, 2-pyrrolidone, N-methylpyrrolidone, 1,3-dimethyl-2-imidazolidinone, gramine, adenine, N,N′-diisopropylethylenediamine, N,N′-bis(2-hydroxyethyl)ethylenediamine, N,N′-dibenzylethylenediamine, and N,N′-diphenylethylenediamine.
Owner:SHOWA DENKO KK
Polishing composition
InactiveCN101496143AFull grinding speedImprove flatnessOther chemical processesSemiconductor/solid-state device manufacturingEthylenediamineEthyl group
Disclosed is a polishing composition which realizes both high planarity and reduction of corrosion in the surface of a wiring metal. Specifically disclosed is a polishing composition containing an oxidizing agent (A), at least one or more acids (B) selected from amino acids, carboxylic acids having 8 or less carbon atoms and inorganic acids, a sulfonic acid (C) having a concentration of not less than 0.01% by mass and an alkyl group having 8 or more carbon atoms, a fatty acid (D) having a concentration of not less than 0.001% by mass and an alkyl group having 8 or more carbon atoms, and at least one or more compounds (E) selected from pyridinecarbonyl compounds, nonionic water-soluble polymers, 2-pyrrolidone, N-methylpyrrolidone, 1,3-dimethyl-2-imidazolidinone, gramine, adenine, N,N'-diisopropylethylenediamine, N,N'-bis(2-hydroxyethyl)ethylenediamine, N,N'-dibenzylethylenediamine and N,N'-diphenylethylenediamine.
Owner:SHOWA DENKO KK
Lithium cell with cathode containing iron disulfide
InactiveUS20090317725A1Improve electrochemical performanceLow viscosityOrganic electrolyte cellsPrimary cell electrodesSlurryPrimary cell
A primary cell having an anode comprising lithium and a cathode comprising iron disulfide (FeS2) and carbon particles. The electrolyte comprises a lithium salt dissolved in a solvent mixture which contains 1,3-dioxolane and preferably 1,3-dimethyl-2-imidazolidinone. A cathode slurry is prepared comprising iron disulfide powder, carbon, binder, and a liquid solvent. The mixture is coated onto a conductive substrate and solvent evaporated leaving a dry cathode coating on the substrate. The anode and cathode can be spirally wound with separator therebetween and inserted into the cell casing with electrolyte then added.
Owner:THE GILLETTE CO
Water-based coating agent composition, water-based lubricating film paint composition comprising same, and member
ActiveCN107429116AInhibition of thickeningPrevent gelationAdditivesEmulsion paintsWater basedEmulsion
The present invention addresses the problem of providing a water-based coating agent composition (in particular, a water-based lubricating film paint composition that includes a solid lubricant) that suppresses the interaction of a curable resin and a surfactant, that suppresses thickening / disproportionation throughout a water-based coating agent, that has superior total fluidity, coating properties, and storage stability, and that can form a favorable coating film, and the problem of providing a member that is provided with said coating film, and a method for forming said coating film. Said problems can be solved by a water-based coating agent composition that includes (A) a curable resin in the form of a water-based emulsion, (B) a surfactant, (C) solid particles, (D) one or more nitrogen-containing heterocyclic compound, and (E) water. In particular, the (C) component preferably includes a solid lubricant, and for reasons of environmental regulations, it is particularly preferable that the (D) component be 1,3-dimethyl-2-imidazolidinone.
Owner:DUPONT TORAY SPECIALTY MATERIALS KK
Liquid crystal orientation agent, liquid crystal orientation film, manufacturing method of liquid crystal orientation film, and liquid crystal display element
InactiveCN103666486AImprove yieldUniform coatingLiquid crystal compositionsNon-linear opticsDiacetone alcoholDiethylene glycol diethyl ether
The invention relates to a liquid crystal orientation agent, a liquid crystal orientation film, a manufacturing method of the liquid crystal orientation film, and a liquid crystal display element. The liquid crystal orientation agent has good printing performance with respect to a substrate and good continuous printing performance and prevents swelling of printing plates. The liquid crystal orientation agent contains at last one polymer (A) selected from the group formed by polyamide acid, polyimide, polyamide acid ester and polysiloxane, a first solvent containing more than one of N-ethyl-2-pyrrolidone, 1,3-dimethyl-2-imidazolone, 3-methoxy-N, N-dimethylpropionamide and so on, and a second solvent containing more than one of propylene glycol diacetate, diethylene glycol diethyl ether, diisoamyl ether diacetone alcohol and so on.
Owner:JSR CORPORATIOON
Method for optical clearing of biological tissues
InactiveCN106556582ATransparent and fastKeep the original shapeFluorescence/phosphorescenceOptical clearingFluorescence
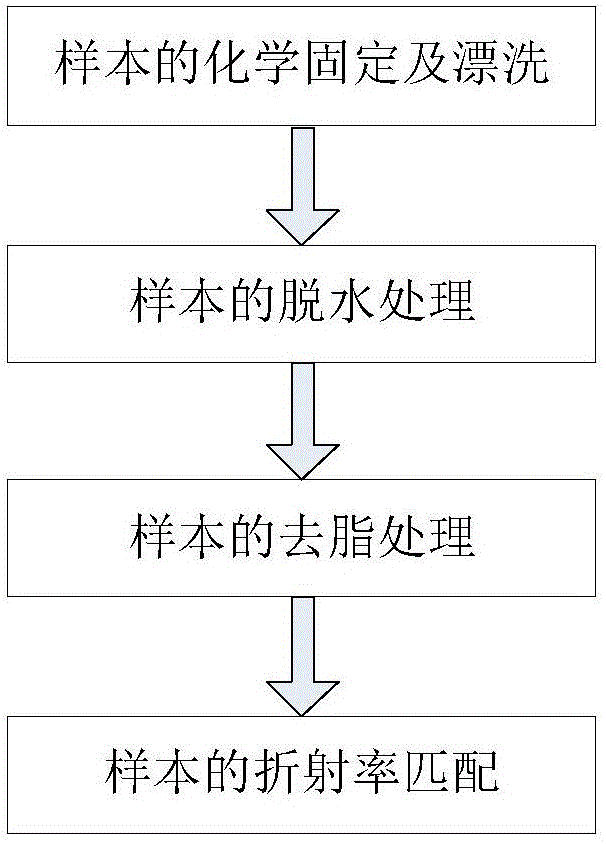
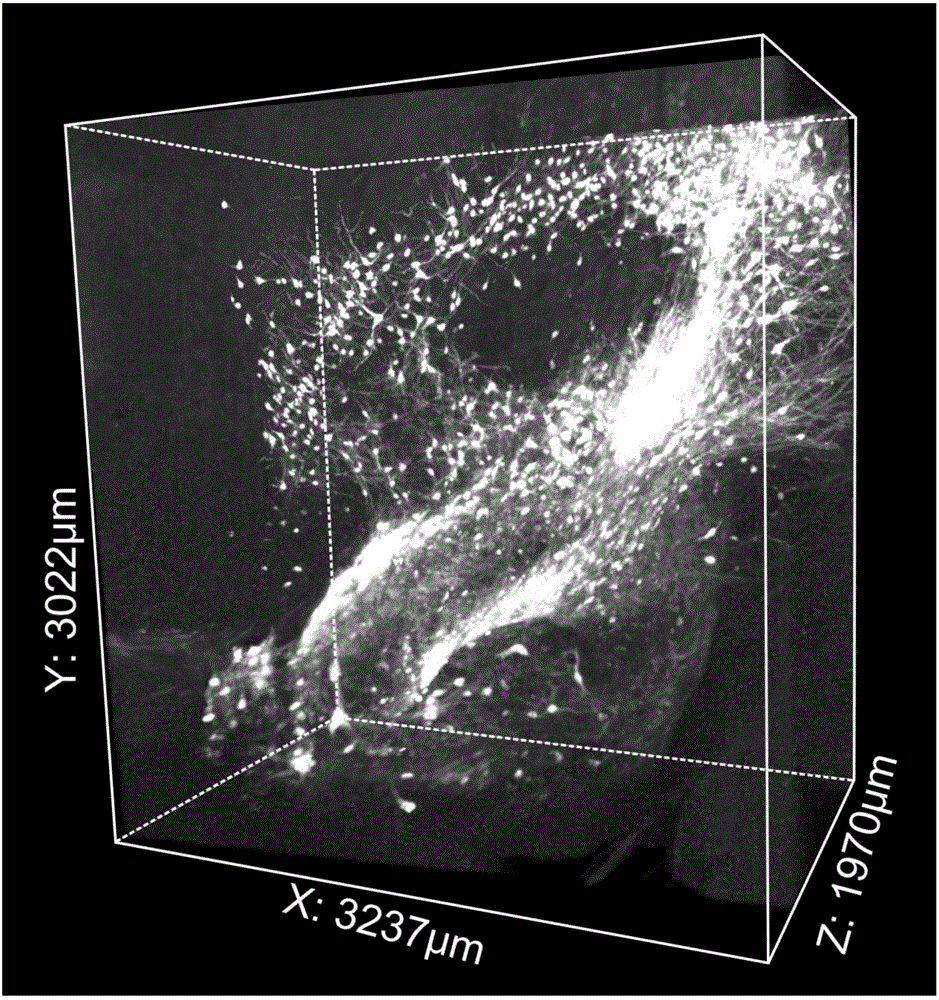
The invention discloses a method for rapid optical clearing of biological tissues. The method includes: employs a strong polar aprotic solvent to conduct degreasing, wherein the strong polar aprotic solvent can be methyl methacrylate, hexamethylphosphoric triamide, N-methyl pyrrolidone, 1, 3-dimethyl-2-imidazolidinone, 1, 4-dioxane or 2, 5-dimethylfuran, then selecting a specific refractive index matching reagent with a refractive index of 1.52-1.59 to conduct clearing treatment, thus realizing rapid clearing of biological tissues. The degreasing and clearing treatment totally need only 18h to realize high clearing of mouse brain tissues, the method not only can realize brain tissue clearing, but also can achieve clearing of various organs, can well maintain the original morphology of biological tissues, and has high clearing degree and repeatability, and good fluorescence retaining effect, the soma and nerve fiber have strong fluorescence signal, and the application range is wide.
Owner:HUAZHONG UNIV OF SCI & TECH
Preparation method for full saccharose polyether polyol
ActiveCN104072748AAdequate responseSolve the problem of poor effect of producing potassium alcoholPolyolPolymer science
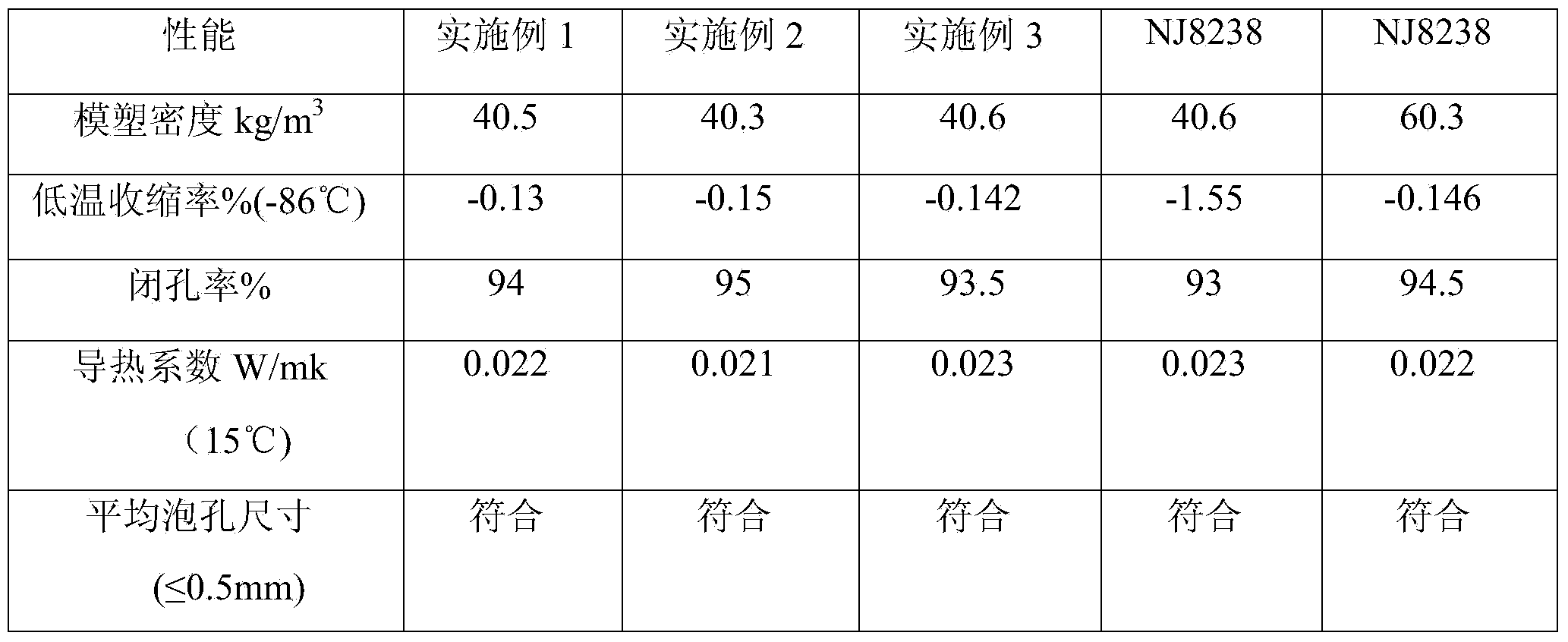
The invention relates to a preparation method for full saccharose polyether polyol, belonging to the technical field of synthesis of polyether polyol. According to the preparation method, only saccharose is taken as an initiator, a catalyst KOH is solvated by 1, 3-dimethyl-2-imidazolone and then is reacted with epoxypropane. The preparation method for full saccharose polyether polyol improves the alcohol and potassium preparation process. The prepared polyether polyol has high degree of functionality and strength, so that the compound material prepared by the polyether polyol can resist low temperature minus 86DEG C when the normal molding density is about 40Kg / m<3>, the overall injection quantity is about 50% lower than that of a normal hard bubble compound polyether system, and the polyether polyol has low cost and high cost performance, and the use field of hard polyurethane foaming plastics is enlarged.
Owner:SHANDONG INOV NEW MATERIALS CO LTD
Process for synthesizing 3,4-difluorobenzonitrile
ActiveCN103539699AProcess raw materials are easy to getShort reaction timeCarboxylic acid nitrile preparationOrganic compound preparationPotassium fluorideChloride
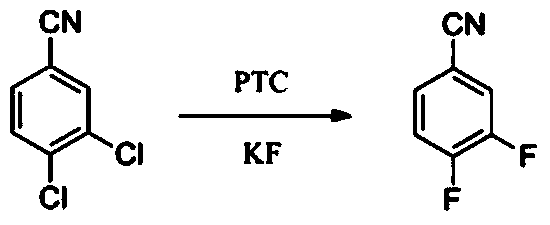
The invention discloses a process for synthesizing 3,4-difluorobenzonitrile. According to the process, 3,4-difluorobenzonitrile with the purity of over 99% is obtained through taking 3,4-dichlorobenzonitrile as a raw material, taking potassium fluoride as a fluorination reagent, taking 1,3-dimethyl-2-imidazolidinone as a reaction solvent, taking bis-(N-bis(dimethylamino)methylene)-iminium chloride as a catalyst, enabling reactants to react for 2-3 hours at the temperature of 130-150 DEG C and react for 5-6 hours at the temperature of 180-200 DEG C, then ending reaction, filtering a reaction solution and then rectifying under reduced pressure, and the yield can reach 85%. Rectification mother liquor (containing the catalyst) is mechanically applied to next-batch reaction directly. According to the process disclosed by the invention, the raw material is easy to obtain, the reaction conditions are mild, the reaction time is short, the operation is simple, the yield is high, the reaction solvent (containing the catalyst) can be repeatedly applied mechanically, the cost is low, and the emission of waste gases, waste water and waste residues is little, so that the process is applicable to industrial production.
Owner:SHANGHAI HUAYI GRP CO
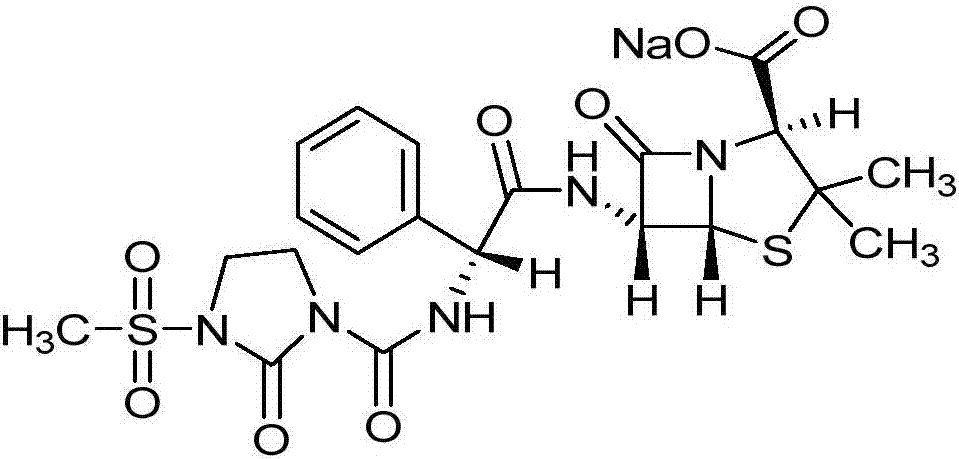
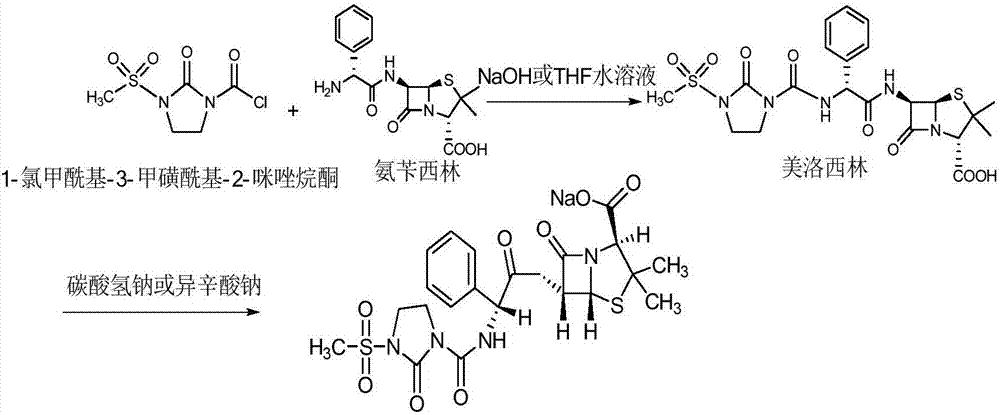
Preparation process of mezlocillin sodium
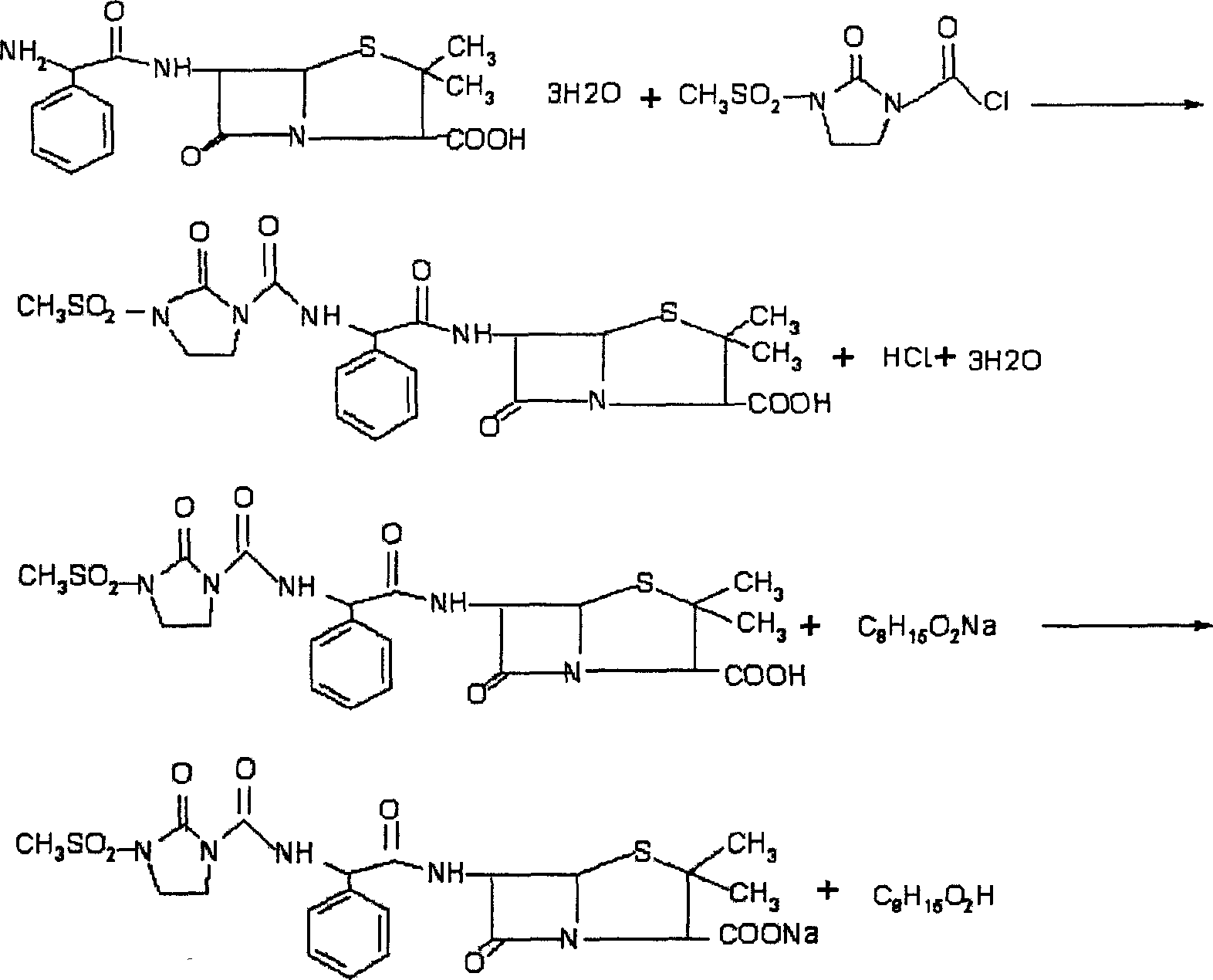
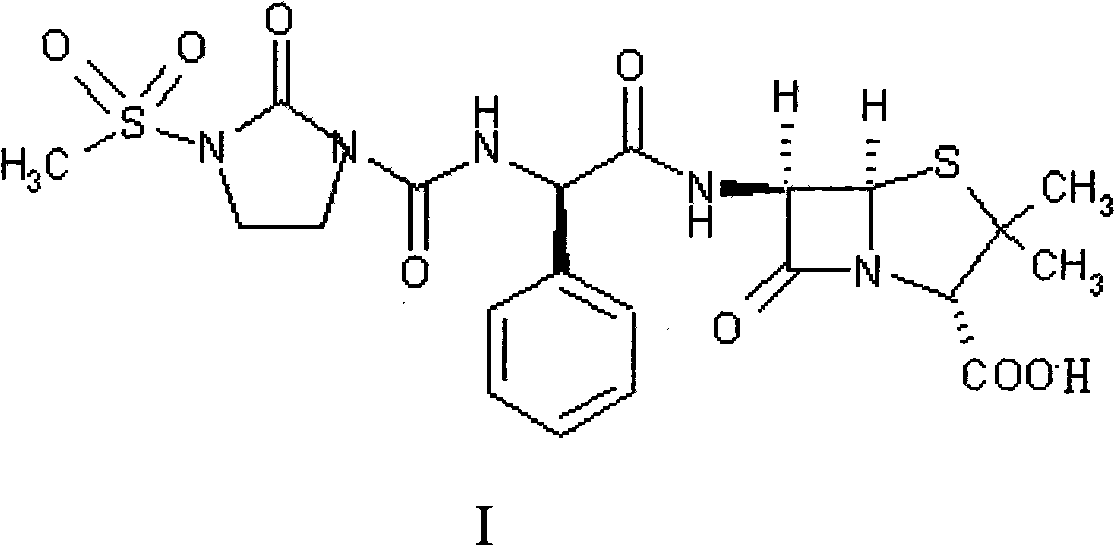
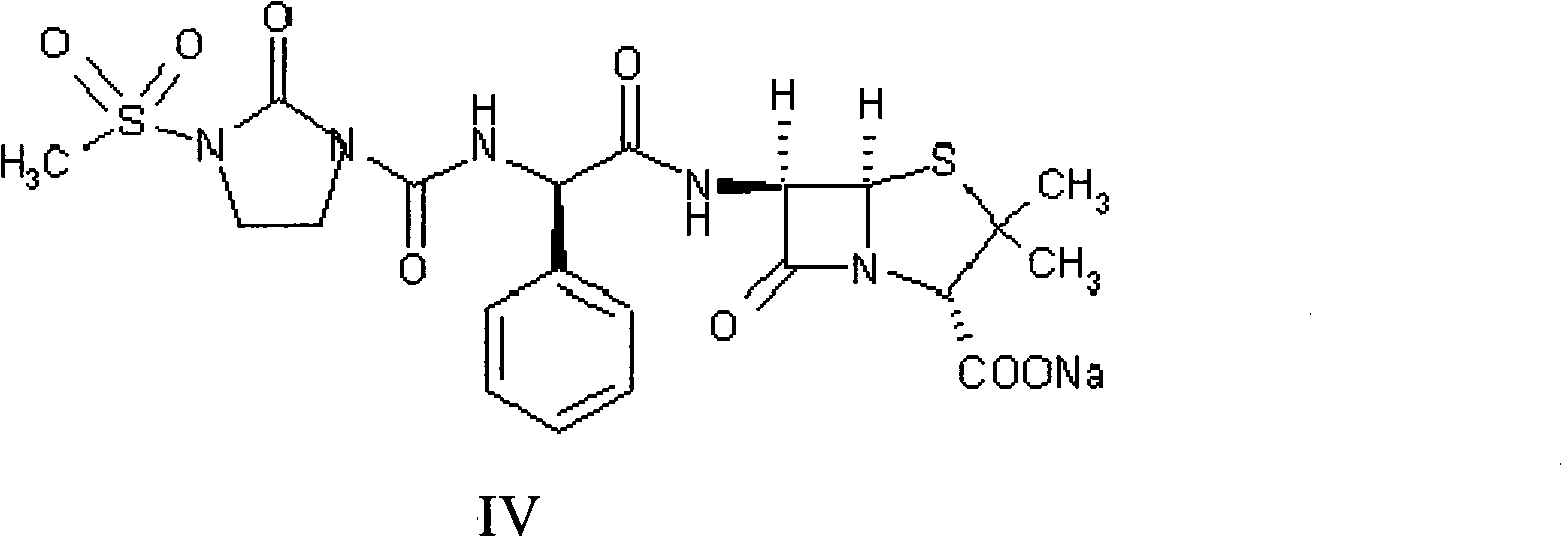
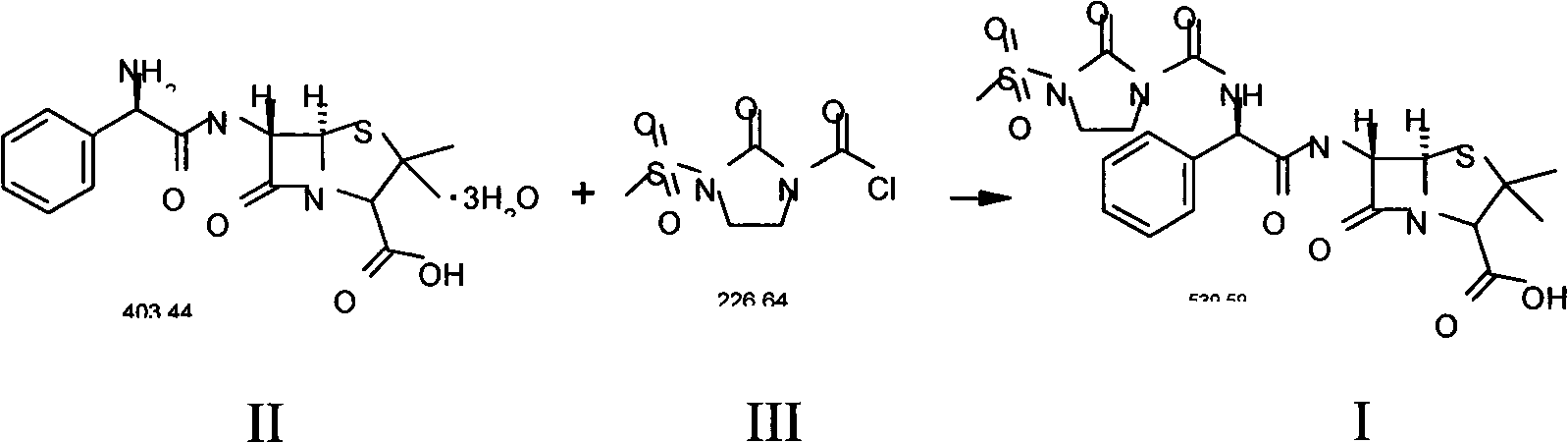
Disclosed is a process for preparing mezlocillin sodium belonging to compound preparation technical field. By acylation reaction of aminobenzyl triaqua acid with 1-chloroformyl-3-mesyl-2-imidazolidinone, acidizing after an ethyl acetate solvent being added, esterified layer removing, salifying reaction after a sodium salt forming agent being added, and seedout, the mezlocillin sodium is obtained. In the invention, the mixed solvent is adopted and the speed of adding the sodium salt forming agent is adjusted. There is recrystallization buffer, so the crystal grain is big and the purity is high.
Owner:REYOUNG PHARMA
Polyamic acid ester liquid crystal alignment agent, and liquid crystal alignment film using same
ActiveCN102893207AImprove interface propertiesReduce surface fine unevennessCoatingsNon-linear opticsMethyl groupThioester
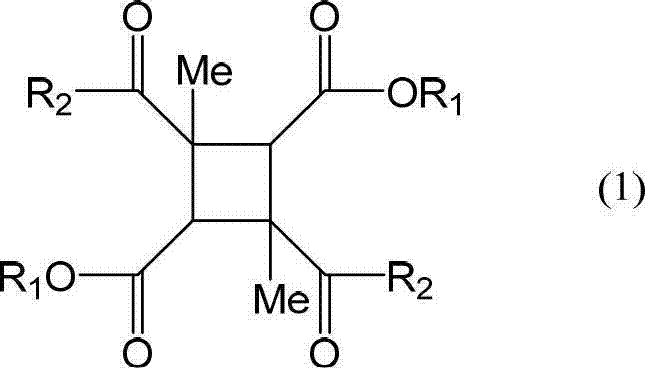
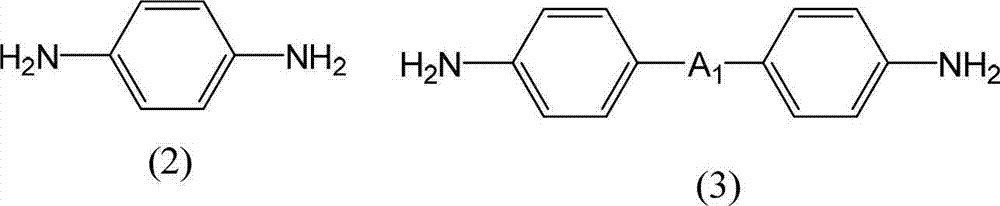
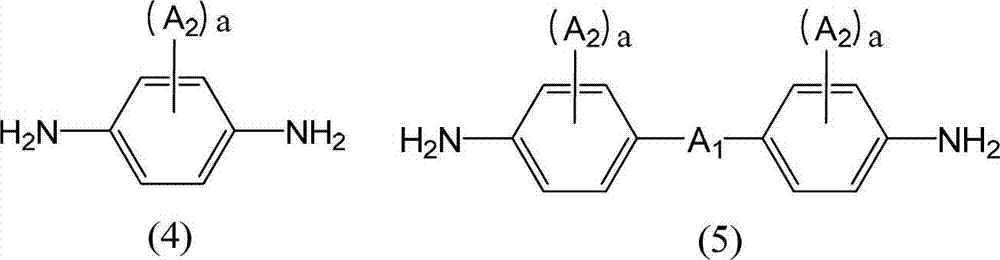
Disclosed is a liquid crystal alignment agent which can reduce microscopic irregularities on the surface of liquid crystal alignment films, improves liquid crystal alignment properties, and has improved electrical characteristics of every kind. The liquid crystal alignment agent contains component (A), component (B) and component (C). Component (A): a polyamic acid ester obtained from a tetracarboxylic acid dialkyl ester derivative containing a tetracarboxylic acid dialkyl ester derivative represented by general formula (1) as at least 60 mol% of the total tetracarboxylic acid dialkyl ester derivative, and a diamine containing at least one diamine selected from the diamines represented by general formulae (2-5). Component (B): a polyamic acid obtained from a tetracarboxylic acid dianhydride, and a diamine. Component (C): a mixed solvent containing at least one type of organic solvent (C1) selected from a group comprising -butyrolactone and derivatives thereof, and at least one type of organic solvent (C2) selected from a group comprising N-methyl-2-pyrrolidone, 1,3-dimethyl-2-imidazolidinone, and derivatives thereof, and in which the content of the organic solvent (C2) is 2%-30% by mass relative to the total amount of both organic solvents (C1, C2). (In general formula (1), R1 is a C1-5 alkyl group, and R2 is a hydroxyl group or a chlorine atom.) (In general formulae (2-5), A1 is a single bond, an ester bond, an amide bond, thioester bond, or a C2-10 divalent organic group, A2 is a halogen atom, a hydroxyl group, an amino group, a thiol group, a nitro group, a phospate group, or a C1-20 monovalent organic group, a is an integer from 1-4, and when a is 2 or higher, the structure of A2 can be the same or different).
Owner:NISSAN CHEM IND LTD
Ink composition, ink set, and image forming method
InactiveUS20130050331A1High temporal stabilityGood rubbing fastnessDuplicating/marking methodsInksSolubilityDouble bond
Disclosed is an ink composition including: (1) a pigment; (2) a polymerization initiator having a content of 2% by mass or more with respect to a total amount of the composition and having a solubility with respect to pure water at 25° C. of 5 to 8 g / l; (3) a compound A that is at least one selected from the group consisting of dimethylacrylamide, diethylacrylamide, N-isopropylacrylamide, dimethylacetamide, N-ethylpyrrolidone, 1-cyclohexyl-2-pyrrolidone, 1-(2-hydroxyethyl)-2-pyrrolidone, 1,3-dimethyl-2-imidazolidinone, tetramethylurea and gamma-valerolactone; (4) a polymerizable compound having two or more ethylenically unsaturated double bonds; and (5) water having a content of 50% by mass or more with respect to the total amount of the composition.
Owner:FUJIFILM CORP
Synthesizing method for 1,3, dimethyl-2-imidazolidinone
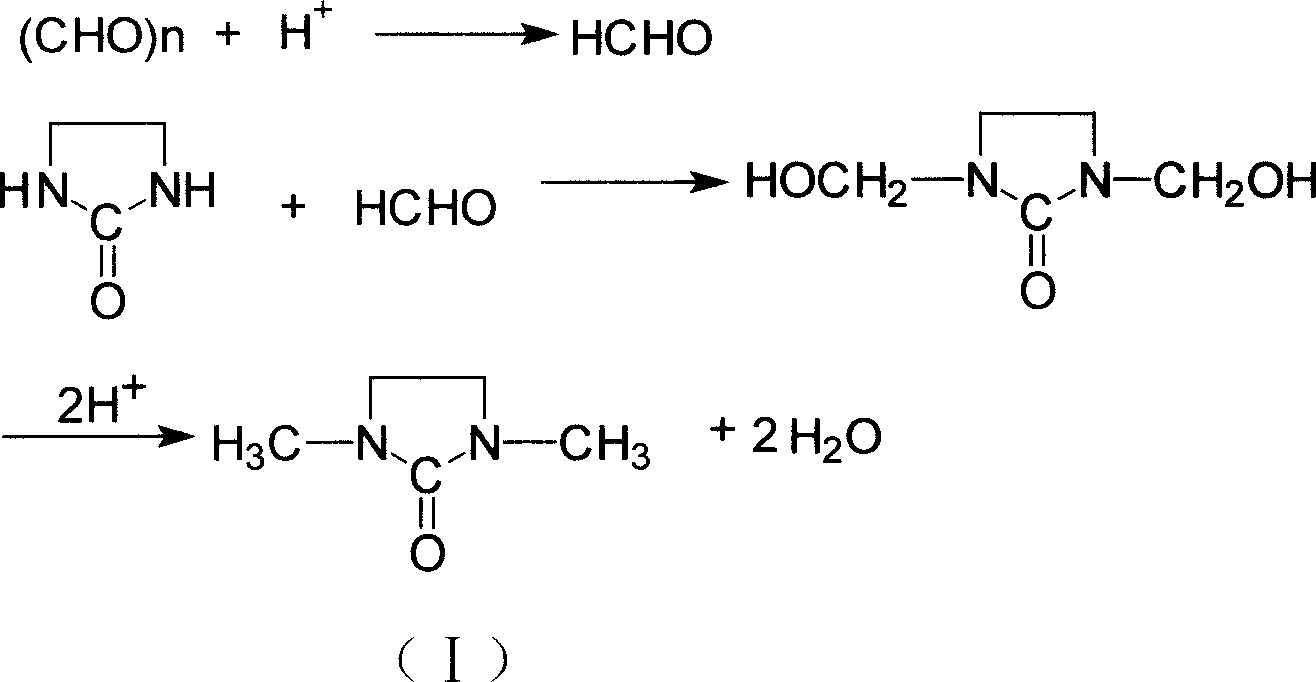
This invention relates to synthetic method of 1, 3 - dimethyl - 2-imidazolidinone. The invention solves the problem in exsiting method that the reaction need 85% concentration methanoic acid as reaction medium, and the generated methanoic acid has no means of reusing. The invention takes paraformaldehyde and 2-imidazolidinone as raw material, 30% to 45%methanoic acid as reaction medium. Firstly proceed paraformaldehyde depolymerization, then add 85%or 94% methanoic acid, warm-up till circumfluence reaction for 12 hours, then gain 1, 3 - dimethyl - 2-imidazolidinone. The invention could cyclic utilize sparse methanoic acid generated from 1, 3 - dimethyl - 2-imidazolidinone synthetic reaction, thereby advance capacity utilization rate, depress manufacturing cost.
Owner:XIAN MODERN CHEM RES INST
Method for manufacturing lithium-contained composite oxide and nonaqueous secondary cell
InactiveCN101707251ASuppress mix-inAvoid decompositionCell electrodesCobalt compounds2-PyrrolidoneWater soluble
The invention provides a method for manufacturing lithium-contained composite oxide represented by a general formula (I) and a nonaqueous secondary cell taking the lithium-contained composite oxide as an anode active substance. LixMyMe1-yO2+Delta (1), wherein M is at least an element selected form Ni, Co and Mn; Me is a metal element different from M; and 0.95<=x<=1.10, 0.1<=y<=1. The compound containing M and Me and the lithium compound are sintered. The sintered substance is cleaned by a cleaning fluid of a water soluble polar non-protonic solvent containing one or two components from N-methyl-2-pyrrolidone (NMP), N,N'-dimethyl-2-imidazolidinone and dimethyl sulfoxide (DMSO).
Owner:PANASONIC CORP
Purification technology of dipyridamole
The invention belongs to the field of drug preparation and particularly relates to a purification technology of a synthetic drug. The method comprises the steps of adding a methanol solvent and a dipyridamole crude product into methyl benzenesulfonic acid and 1-(3-aminobenzene)-3-methyl-2-imidazolone, stirring for reaction to form a sulfonate intermediate product, mixing the sulfonate intermediate product and a 70% methanol solution for dissolving, adding alkaline liquor to regulate pH (potential of hydrogen) of a system to 8-8.5, then adding active carbon, carrying out heating, decoloring, stirring and press filtration, cooling filtrate, and carrying out centrifuge, washing and drying to form purified high-purity dipyridamole.
Owner:常州康普药业有限公司
Process for production of polyimide film, and polyamic acid solution composition
ActiveCN101802059AHigh tensile strengthIncrease elasticityCoatingsN-ethyl-2-pyrrolidone2-Pyrrolidone
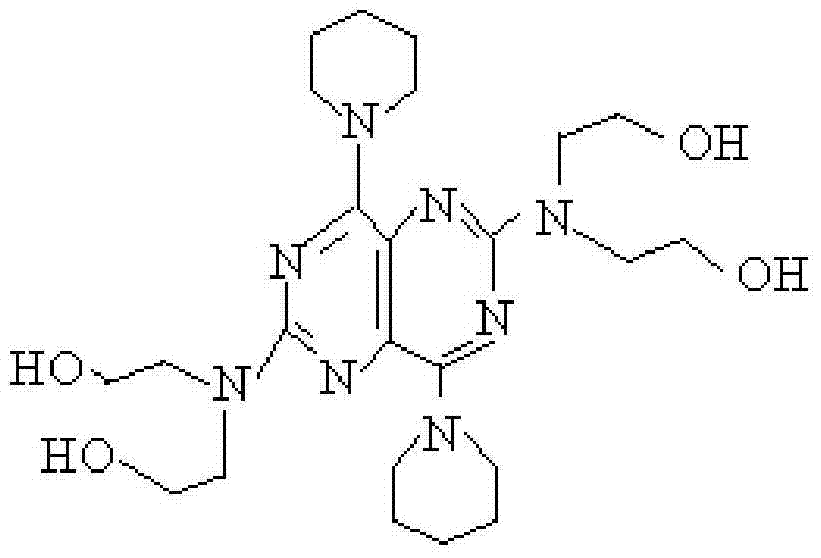
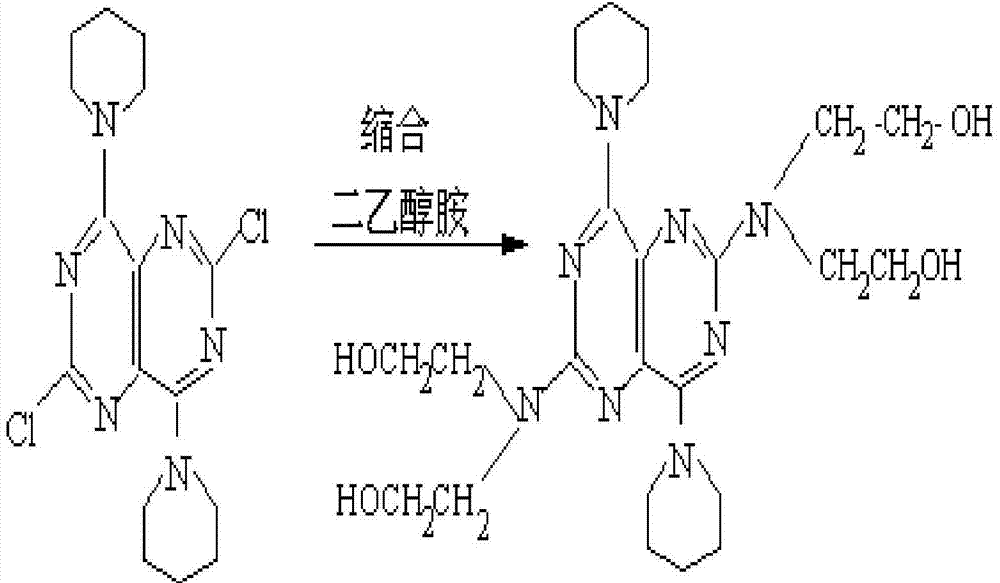
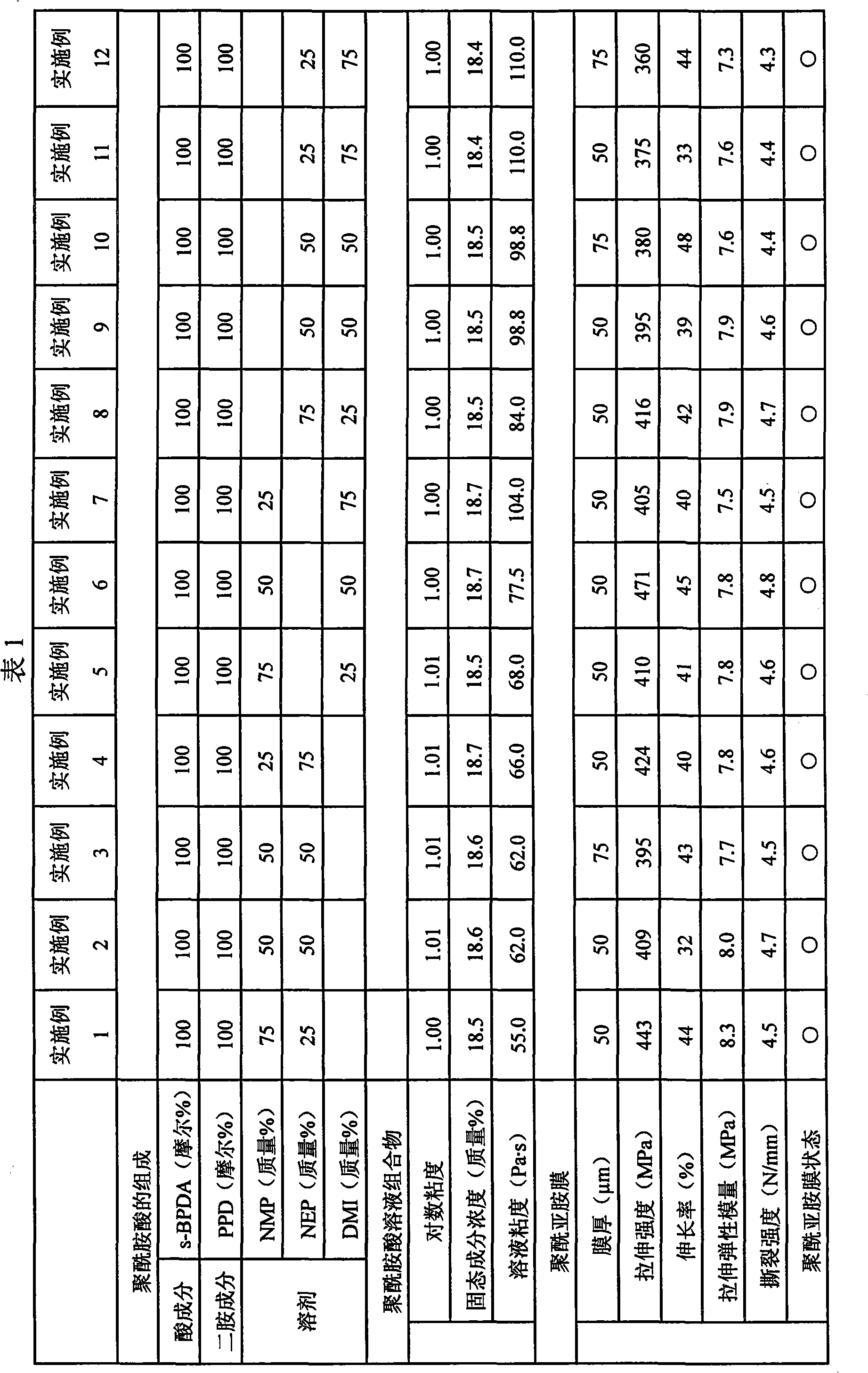
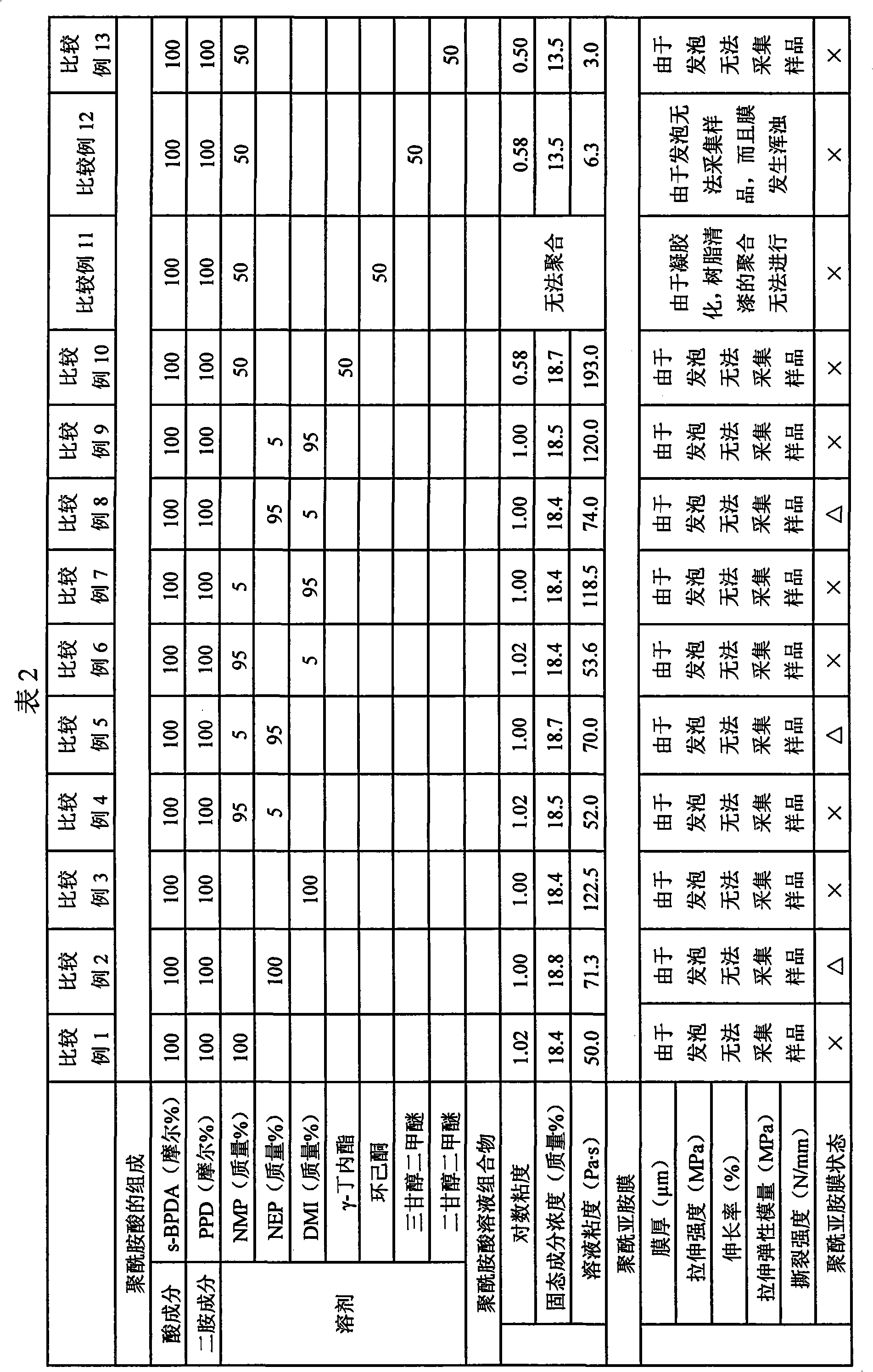
Disclosed is a process for producing a polyimide film, which comprises applying a polyamic acid solution composition to a base material to form a coating film, and subsequently heating the coating film, wherein the polyamic acid solution composition comprises a mixed solvent of two or more solvents selected from N-methyl-2-pyrrolidone, N-ethyl-2-pyrrolidone and 1,3-dimethyl-2-imidazolidinone (wherein each of the solvents is contained in an amount of 7 to 93 mass% relative to 100 mass% of the total amount of the solvents) and a polyamic acid mainly composed of s-BPDA and PPD. The process enables to produce a polyimide film having a film thickness of more than 40 [mu]m without the need of expanding the film.
Owner:UBE IND LTD
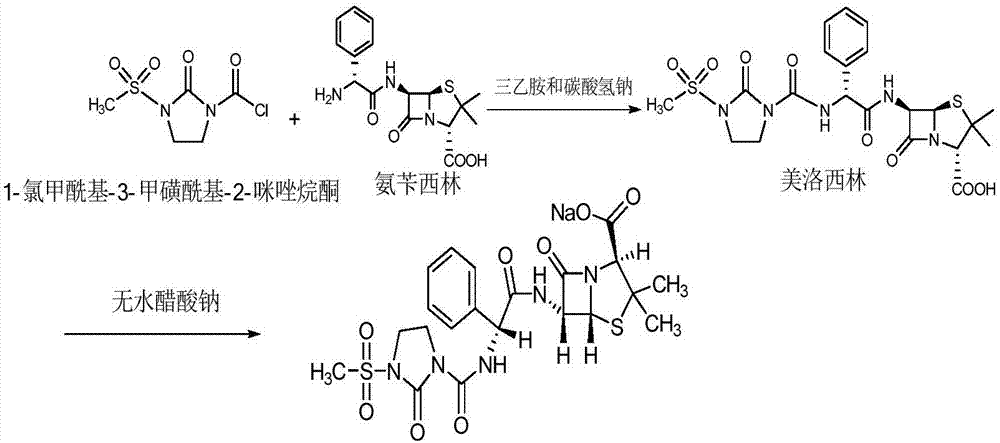
Preparation process of Mezlocillin
The invention relates to a preparation process of Mezlocillin, which reacts Ampicillin trihydrate with 1-chlorocarbonyl-3-methylsulfonyl-2-imidazolidinone in water phase under alkaline condition and then proceeds acidified crystallization, filtration, washing and drying in acetone / water, or alcohol / water, or isopropanol / water system to obtain Mezlocillin. The Mezlocillin prepared by the inventive method can reach 95%-97% of molar yield, 0.06%-0.4% of organic residual quantity, more than 98.5% of dry basis content, less than 0.4% of single impurity, less than 1.5% of total related substances and good quality, is particularly suitable for direct lyophilization in water-phase salt formation to obtain high-quality Mezlocillin sodium for injection of medicinal product, and has prominent economical and social benefits.
Owner:ZHEJIANG JINHUA CONBA BIO PHARM CO LTD
Polyamide-imide resin composition for lubricating coating material
ActiveCN104471000AImprove heat resistanceHigh mechanical strengthCoatingsBase-materialsSolubilityGlass transition
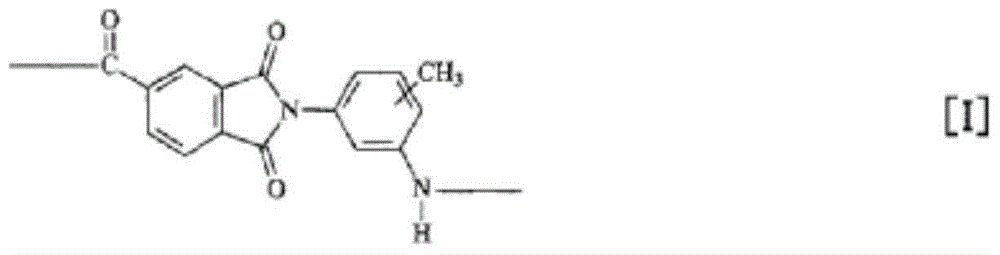
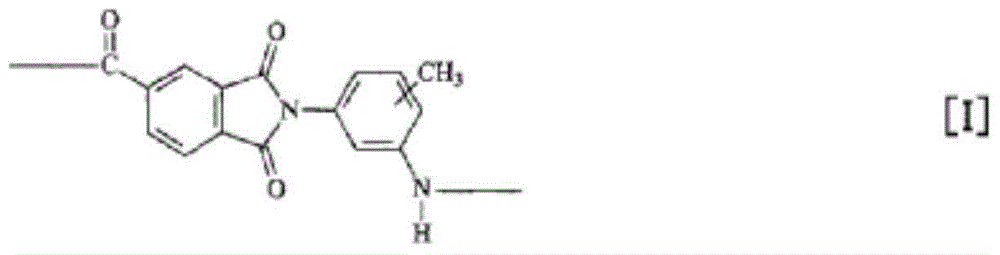
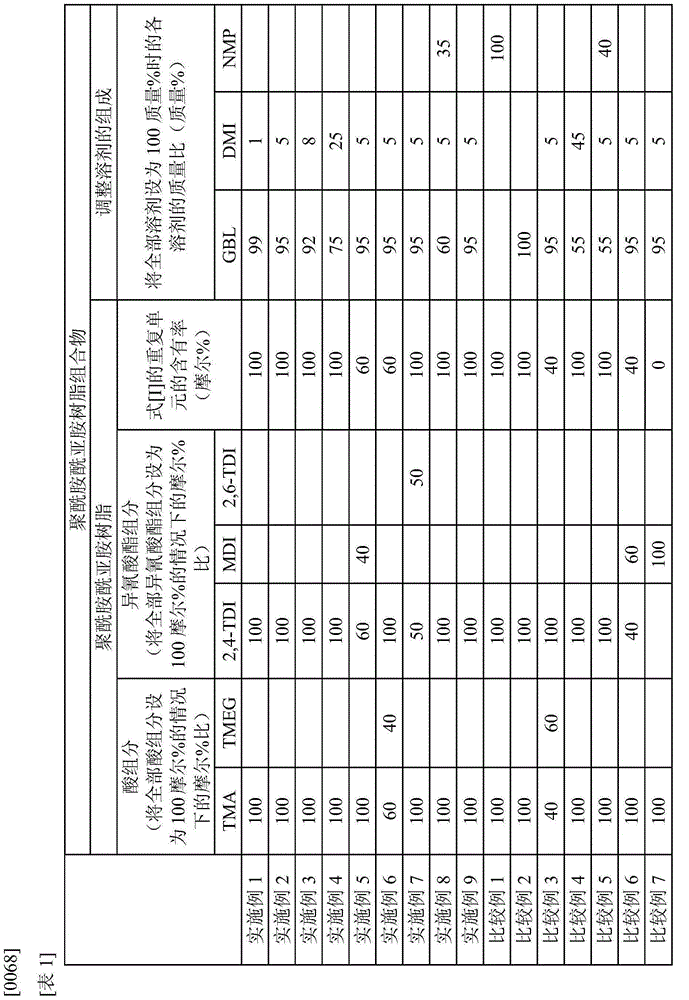
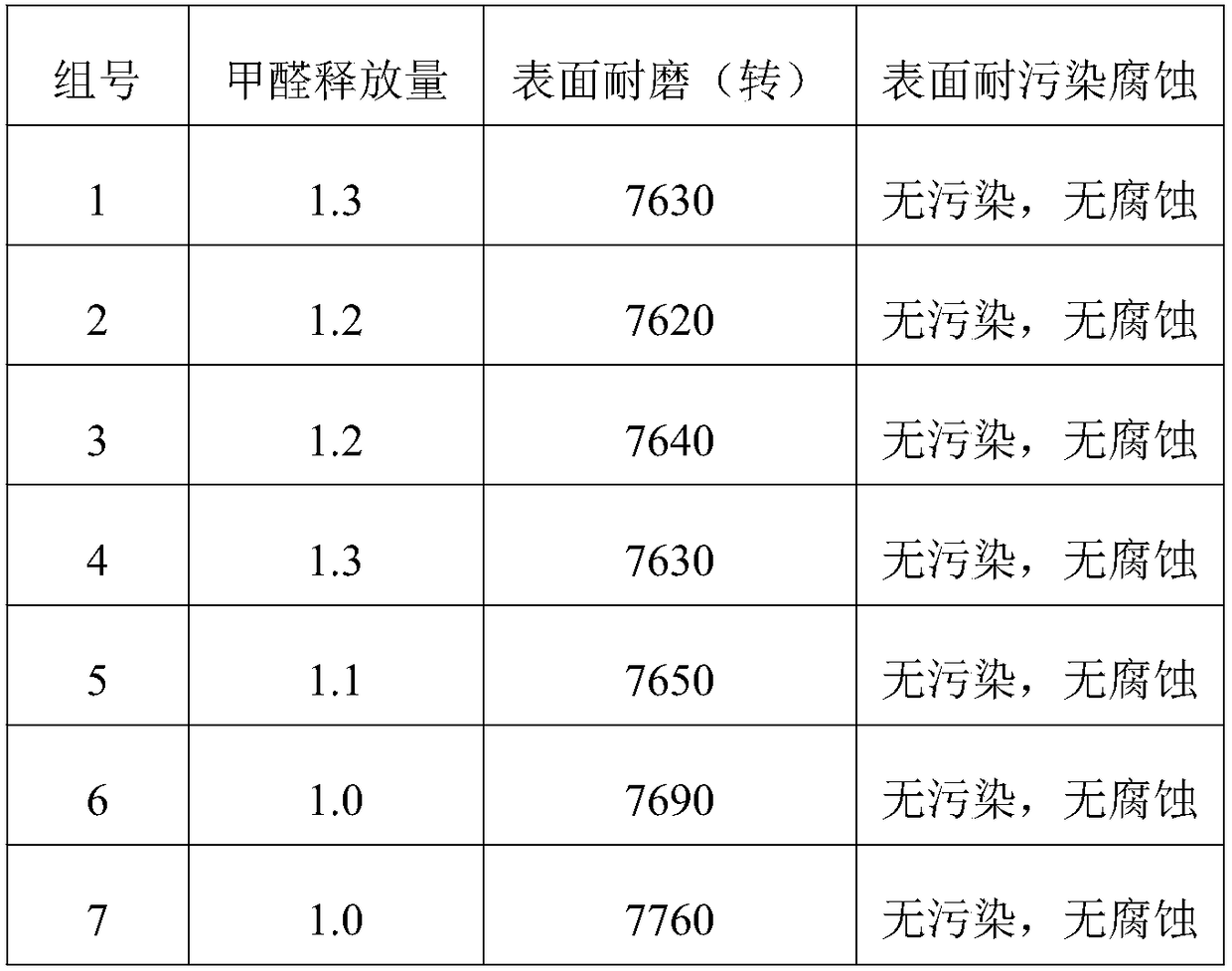
Provided is a polyamide-imide resin composition for lubricating coating materials which retains the excellent heat resistance (i.e., high glass transition temperature) and mechanical strength (i.e., high modulus) inherent in polyamide-imide resins and in which the resin has excellent solubility in the solvents including gamma-butyrolactone, which is a non-amide solvent, and the dissolved state can be maintained for a long time even when the solvents are in the state of being apt to easily absorb moisture. The polyamide-imide resin composition for lubricating coating materials is characterized by containing a polyamide-imide resin that contains the repeating unit represented by formula [I] in an amount of 50 mol% or more of all the repeating units contained in the structure of the polyamide-imide resin and by containing gamma-butyrolactone and 1,3-dimethyl-2-imidazolidinone as solvents.
Owner:TOYOBO CO LTD
Nano oxidation aldehyde-free floor, production method thereof, nano oxidation aldehyde-eliminating liquid and eucalyptus-rosemary nano aldehyde-eliminating liquid
ActiveCN108410271APlay a synergistic roleStrong adhesionOther plywood/veneer working apparatusAntifouling/underwater paintsIonROSEMARY OIL
The invention belongs to the technical field of aldehyde elimination, and provides a nano oxidation aldehyde-free floor, a production method thereof, a nano oxidation aldehyde-eliminating liquid and aeucalyptus-rosemary nano aldehyde-eliminating liquid. The nano oxidation aldehyde-eliminating liquid is prepared by compounding modified chitosan, an osmotic agent, 2-imidazolidinone, nano zinc oxide, nano silicon oxide, and deionized water according to a certain ratio; and has the advantages of strong adhesion force, excellent permeability, good aldehyde-eliminating effect, and long acting effect. The eucalyptus-rosemary nano aldehyde-eliminating liquid is prepared by compounding the nano oxidation aldehyde-eliminating liquid, eucalyptus leaf oil, and rosemary oil according to a certain ratio and is capable of reducing released formaldehyde, killing bacteria and mites, and preventing mold. By adding the eucalyptus-rosemary nano aldehyde-eliminating liquid during the process of hot-pressing a fiber board and decorative paper, the amount of released formaldehyde is reduced, and at the same time, the product has the functions of killing bacteria and mites, and preventing mold. Furthermore, nano oxidation aldehyde-eliminating liquid is sprayed on or roller-painted on the surface, back face, groove and tongue of a product to further control the amount of released formaldehyde.
Owner:成都市美康三杉木业有限公司
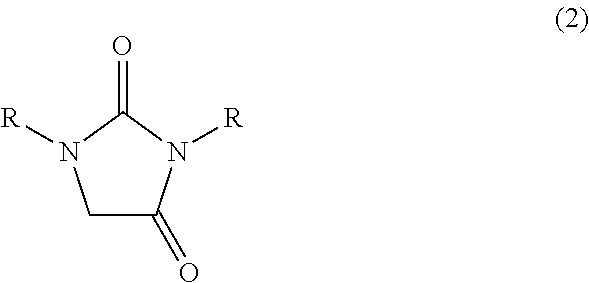
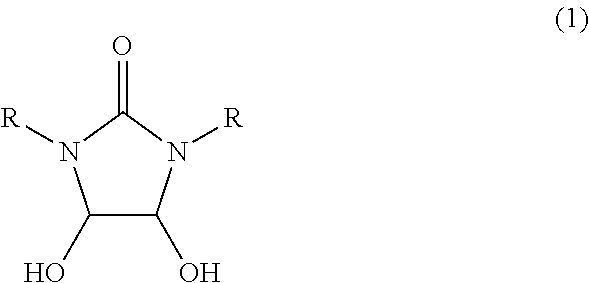
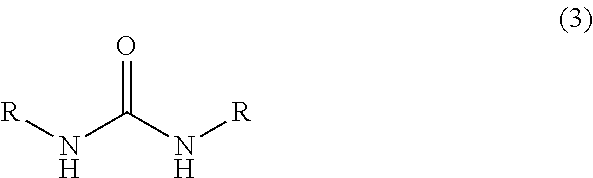
Process for preparing imidazolidin-2,4-dione compound and method for acquiring solid state 4,5-dihydroxy-2-imidazolidinone compound
InactiveUS20100010233A1Safe and simply and easy to prepareHigh yieldOrganic chemistryOrganic solventGlyoxal synthesis
An object of the present invention is to provide an industrially suitable process for preparing an imidazolidin-2,4-dione compound which is safe, simple and easy to prepare the imidazolidin-2,4-dione compound with high yield, which is a useful compound, for example, as a material for decomposing a harmful halogenated aromatic hydrocarbon compound such as dioxin, etc., an electroless silver plating solution for electronic parts, or a diazo copying material, etc. This object can be solved by a process for preparing an imidazolidin-2,4-dione compound which comprises subjecting a 4,5-dihydroxy-2-imidazolidinone compound, (1) to dehydration reaction in the presence of an acid catalyst(s); or (2) to reaction at 100 to 300° C. Or, it can be solved by a process for preparing an imidazolidin-2,4-dione compound which comprises reacting a mixed solution of a urea compound, glyoxal, and a base at 20 to 300° C.Also, an object of the present invention is to provide a method for acquiring a 4,5-dihydroxy-2-imidazolidinone from an aqueous solution containing a 4,5-dihydroxy-2-imidazolidinone by a simple and easy method. This object can be solved by a process for acquiring a solid state 4,5-dihydroxy-2-imidazolidinone compound which comprises mixing an organic solvent with an aqueous solution containing a 4,5-dihydroxy-2-imidazolidinone compound, and subjecting the mixture to azeotropic distillation.
Owner:UBE IND LTD
Emulsifiable concentrate composition
InactiveUS20110009271A1Stable storageSatisfactory emulsion stabilityBiocideOrganic active ingredientsSolventGamma-Butyrolactone
Disclosed is an emulsifiable concentrate composition characterized by containing not less than 0.5% by weight but not more than 25% by weight of a hydrophobic agrochemically active compound, not less than 5% by weight but not more than 15% by weight of a surfactant, not less than 2% by weight but not more than 60% by weight of an aromatic hydrocarbon solvent, not less than 2% by weight but not more than 60% by weight of γ-butyrolactone, and not less than 12% by weight but not more than 90% by weight of 1,3-dimethyl-2-imidazolidinone. The emulsifiable concentrate composition is also characterized in that the weight ratio of 1,3-dimethyl-2-imidazolidinone to γ-butyrolactone, namely (1,3-dimethyl-2-imidazolidinone):(γ-butyrolactone) is within the range from 1:0.03 to 1:2.
Owner:SUMITOMO CHEM CO LTD
Polymeric intumescent flame retardant and preparation method thereof
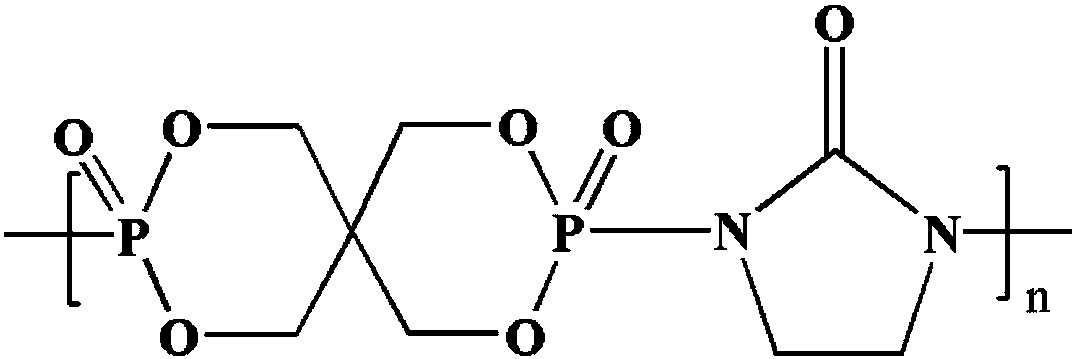
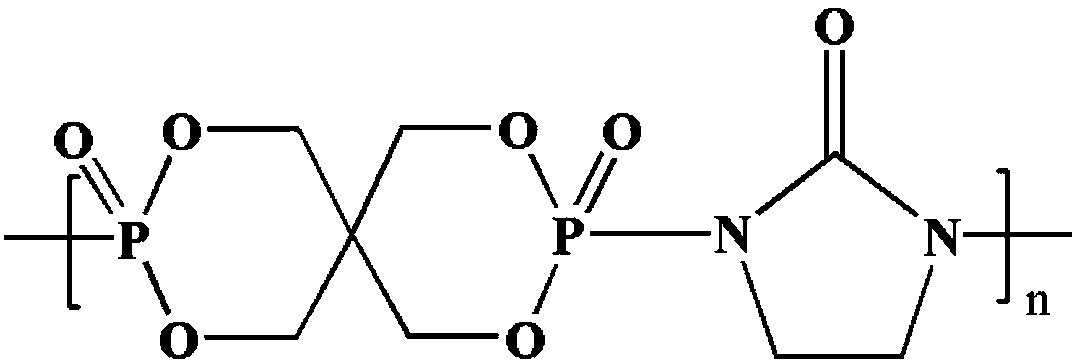
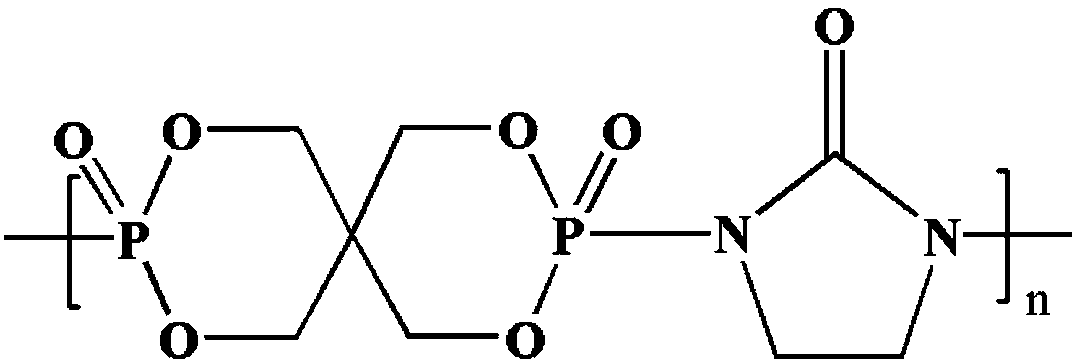
InactiveCN109096492AHigh molecular weightNarrow molecular weight distributionPolymer scienceFiltration
The invention relates to a polymeric intumescent flame retardant and a preparation method thereof and belongs to the technical field of phosphorus-containing flame retardants. The structural formula is as shown in the description. In the formula, the polymerization degree n is an integer of 5-200. The preparation method comprises the following steps: adding pentaerythritol and just distilled phosphorus oxychloride into a solvent A under inert gas shielding, mixing at a room temperature until the system is stabilized, slowly raising the temperature until no hydrogen chloride gas is released, washing, performing suction filtration, and drying to obtain an intermediate spironophosphoryl dichloride; adding 2-imidazolidinone into a solvent B, stirring until the solution is clarified and transparent, adding the intermediate spironophosphoryl dichloride, stirring until the system is uniformly mixed, reacting at a temperature of 50 DEG C for 2 hours, adding an acid-binding agent, washing afterreaction completion, performing suction filtration, and drying, thereby obtaining the polymeric intumescent flame retardant. The polymeric intumescent flame retardant provided by the invention is high in molecular weight, narrow in molecular weight distribution and excellent in compatibility with the matrix, and has excellent heat stability and high flame-retardant efficiency.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Liquid agrochemical composition containing hydrophobic agrochemically active compound
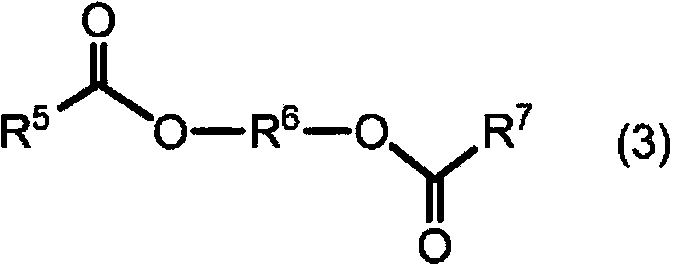
There is provided a liquid agrochemical composition having a stable water-diluted state, which comprises 0.5 to 30% by weight of one or more hydrophobic agrochemical active compounds, 1 to 20% by weight of one or more nonionic surfactants selected from the group consisting of a polyoxyethylene polyoxypropylene block copolymer and the like, 0 to 10% by weight of one or more anionic surfactants, 6 to 60% by weight of an ether compound represented by R<1>-O-A<1>-O-A<2>-(O-A<3>)n-O-R<2>, wherein R<1 >and R<2 >represent a C1-3 alkyl group, A<1>, A<2 >and A<3 >represent an ethylene group or a propylene group, and n represents 0, 1 or 2, and 20 to 75% by weight of 1,3-dimethyl-2-imidazolidinone.
Owner:SUMITOMO CHEM CO LTD
Nonaqueous ink-jet ink and ink-jet recording method
InactiveUS20090011264A1Easy to useGood printing adaptabilityDuplicating/marking methodsSynthetic resin layered productsCompound (substance)Solvent
A nonaqueous ink-jet ink comprising at least solvents (A) and (B) cited below, a pigment, and a polymer compound, wherein the content of solvent (A) is 3-15% by weight based on the total weight of the ink-jet ink, and the content of solvent (B) is 50-90% by weight: Solvent (A): 1,3-dimethyl-2-imidazolidinone; Solvent (B): a solvent comprising at least one compound selected from the group consisting of the compounds represented by following Formulas (1) and (2): Formula (1) R1—(OX1)2—O—R2, and
Owner:KONICA MINOLTA IJ TECHNOLOGIES INC
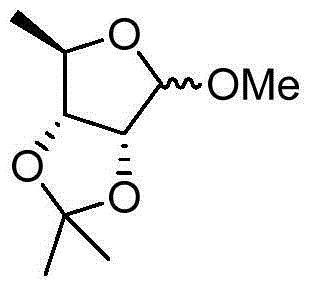
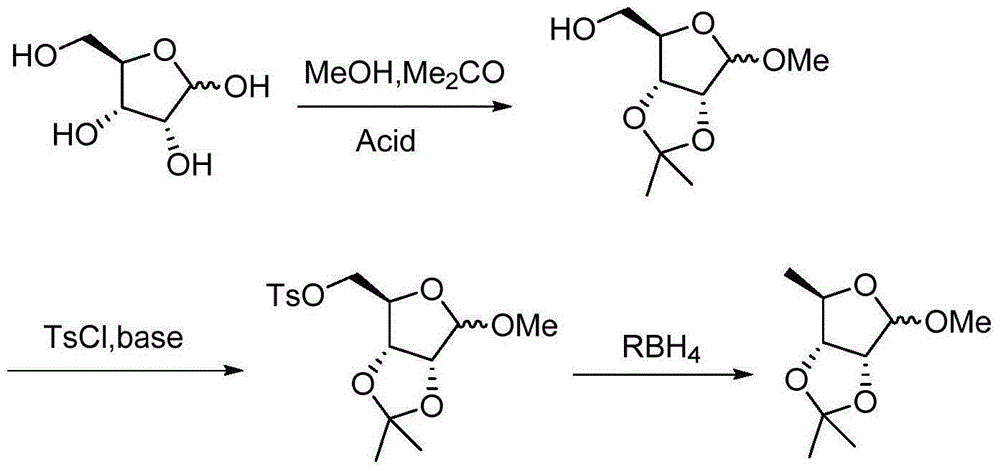
Preparation method of methyl-2,3-O-isopropylidene-5-deoxy-D-ribofuranoside
InactiveCN105037453ANo purification requiredMild conditionsSugar derivativesSugar derivatives preparationProper treatmentReaction intermediate
The invention discloses a preparation method of methyl-2,3-O-isopropylidene-5-deoxy-D-ribofuranoside. The method comprises: taking D-ribose as an initial material to obtain methyl-2,3-O-isopropylidene-D-ribofuranoside under the protection of methanol / acetone; reacting methyl-2,3-O-isopropylidene-D-ribofuranoside with paratoluensulfonyl chloride to obtain methyl-2,3-O-isopropylidene-5-O-paratoluenesulfonyl-D-ribofuranoside in alkaline condition, wherein the reaction is subjected to proper treatment and needs no purification; taking hexamethylphosphoric triamide or N,N-dimethyl-2-imidazolidinone as a reducing solvent; and using hydroboron for reduction to obtain methyl-2,3-O-isopropylidene-5-deoxy-D-ribofuranoside. The reducing reaction agent can be recycled; the reaction intermediates are subjected to proper treatment and need no purification; and the preparation method is simple to operate, wild in condition, and low in production cost.
Owner:启东东岳药业有限公司
Synthesis method of 1,3-dimethyl-2-imidazolone
The invention discloses a synthesis method of 1,3-dimethyl-2-imidazolone. The method comprises steps as follows: (1) montmorillonite, palladium on carbon, 2-imidazolidone and a formaldehyde water solution are sequentially added to a heatable and inflatable autoclave, and hydrogen is introduced into the autoclave for a reaction at the temperature of 110-160 DEG C for 1-5 h; (2) a material obtainedin the step (1) is filtered to recover montmorillonite and palladium on carbon, remaining formaldehyde and by-product water in filtrate are removed through distillation after the filtrate is collected, and a crude product is obtained; (3) the crude product is purified by rectification, and 1,3-dimethyl-2-imidazolone is obtained. The used catalyst is montmorillonite which is low in cost, can be directly used without further treatment or modification and is easy to recycle.
Owner:XIAMEN UNIV
Method for preparing sertindole by using alkyl imidazole type ionic liquid as solvent
InactiveCN101899036ASolving Recycling ProblemsSolving Purification ProblemsOrganic chemistryOrganic synthesisSolvent
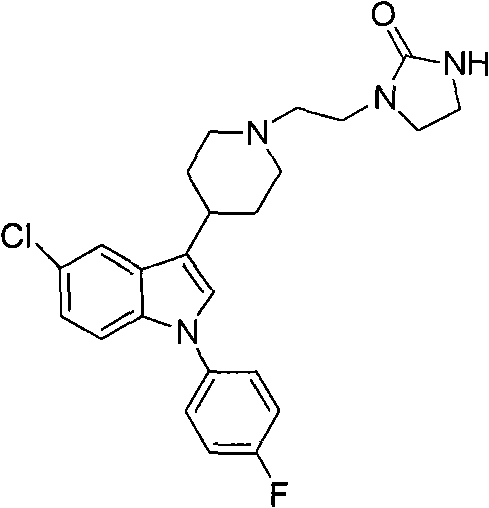
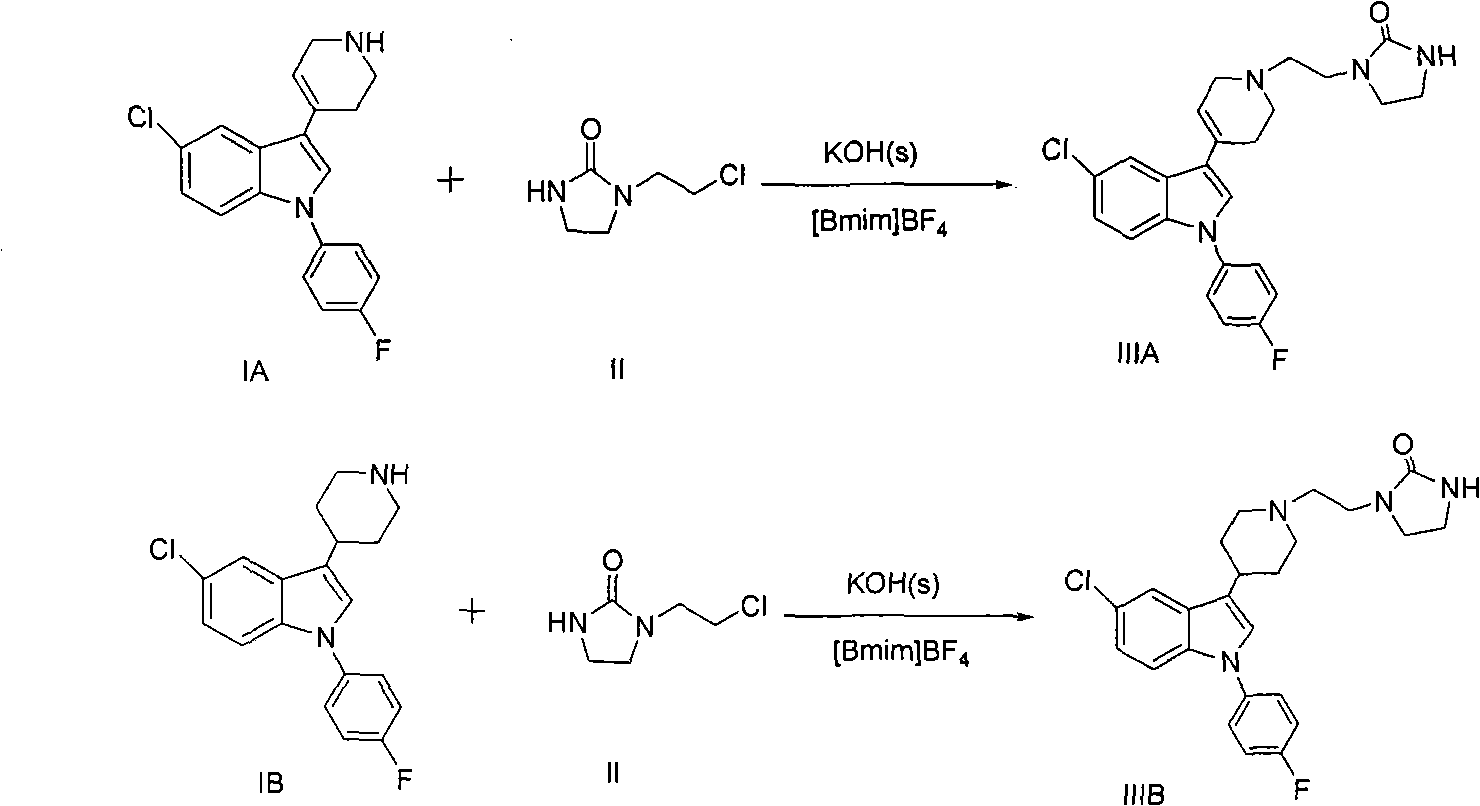
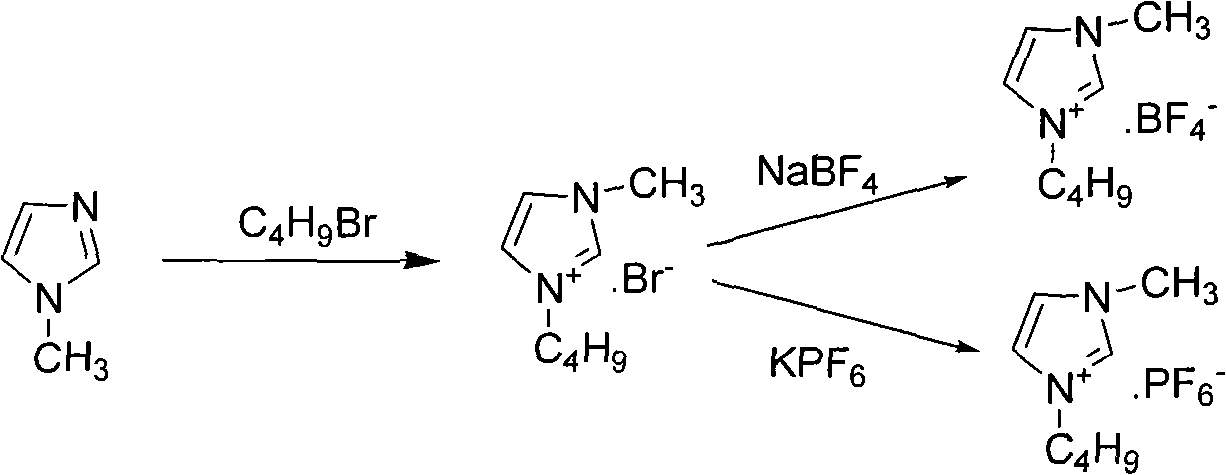
The invention discloses a method for preparing sertindole, which belongs to the technical field of organic synthesis. The method comprises the following steps of: using alkyl imidazole type ionic liquid as a solvent; and under the catalysis of a solid base, reacting 5-chloro-1-(4-fluorophenyl)-3-(1,2,3,6-tetrahydropyridine-4-yl)-1H-indole (IA) or a salt thereof, or 5-chloro-1-(4-fluorophenyl)-3-(4-piperidinyl)-1H-indole (IB) or a salt thereof with 1-(2-chloroethyl)-2-imidazole azululanone (II) to generate 1-[2-[4-[5-chloro-1-(4-fluorophenyl)-1-H-indole-3-yl]-5,6-dihydro-piperidine-1(2H)-yl]ethyl]-2-imidazolidinone (IIIA) or 1-[2-[4-[5-chloro-1-(4-fluorophenyl)-1-H-indole-3-yl]-1-piperidinyl]ethyl]-2-imidazolidinone (IIIB, sertindole). The method can prepare the sertindole and a structuralanalog thereof with high selectivity and high yield, the purity of the prepared sertindole is over 99.5 percent, and the yield can reach 85 to 90 percent. The method is simple and convenient to operate, has an easily controllable reaction condition and high selectivity, is environment-friendly, and is suitable for industrial production.
Owner:ZHENGZHOU UNIV
Lithium cell with cathode containing iron disulfide
InactiveCN102067358ASignificant outgassingImprove conductivityOrganic electrolyte cellsPrimary cell electrodesSlurrySolvent
A primary cell having an anode comprising lithium and a cathode comprising iron disulfide (FeS2) and carbon particles. The electrolyte comprises a lithium salt dissolved in a solvent mixture which contains 1,3-dioxolane and preferably 1,3-dimethyl-2-imidazolidinone. A cathode slurry is prepared comprising iron disulfide powder, carbon, binder, and a liquid solvent. The mixture is coated onto a conductive substrate and solvent evaporated leaving a dry cathode coating on the substrate. The anode and cathode can be spirally wound with separator therebetween and inserted into the cell casing with electrolyte then added.
Owner:THE GILLETTE CO
Organochlorine content determination method
The invention provides an organochlorine content determination method. The method comprises the following steps: weighing a pure chlorohydrocarbon compound contained in chemical engineering products, and diluting by using a solvent to form a certain concentration of a solution, wherein the solvent can be one or more of water, N,N-dimethyl formamide, dimethyl sulfoxide, 1,3-dimethyl-2-imidazolone and liquid paraffin; and respectively adding standard solutions into sample bottles, sealing by using a sealer, putting the sample bottles in a headspace sample cell, setting gas chromatographic operation parameters, and testing by adopting a gas chromatographic static headspace technology. The method for determining the content of organochlorine in the chemical engineering products by using gas chromatography has the advantages of high sensitivity, simple operation, and elimination of test errors brought by manual operation due to the adoption of the static headspace technology.
Owner:SHANDONG DAMING FINE CHEM CO LTD
Preparation technology of mezlocillin sodium
The invention relates to a preparation technology of mezlocillin sodium, and belongs to the technical field of drug synthesis. The preparation technology comprises the steps of performing an acylation reaction on ampicillin trihydrate and 1-chloroformyl-3-mesyl-2-imidazolidinone under a low-temperature alkaline condition, performing decoloration, filtering, acidification, washing and dehydration, adding sodium salt, and then performing crystallization to obtain mezlocillin sodium, wherein ampicillin needs to be dissolved and clarified by triethylamine prior to the acylation reaction; and 1-chloroformyl-3-mesyl-2-imidazolidinone and an ethyl acetate solvent are mixed and then added to a reaction system twice. According to the preparation technology, a dissolution manner of ampicillin trihydrate is changed and an addition manner of 1-chloroformyl-3-mesyl-2-imidazolidinone is changed, so that a side reaction in a reaction process is reduced; the washing is performed by purified water after the acidification and anhydrous sodium acetate is used during the crystallization, so that impurities are further reduced; compared with an original drug, a prepared product has the advantages in the quantity and levels of the impurities.
Owner:REYOUNG PHARMA
Emulsifiable concentrate composition
InactiveUS20100331186A1Stable storageMaintain good propertiesPlant growth regulatorsBiocideEtherSolvent
Disclosed is an emulsifiable concentrate composition characterized by containing not less than 0.5% by weight but not more than 25% by weight of a hydrophobic agrochemically active compound, not less than 5% by weight but not more than 15% by weight of a surfactant, not less than 2% by weight but not more than 60% by weight of an aromatic hydrocarbon solvent, not less than 2% by weight but not more than 60% by weight of an ether compound represented by the following formula (1): R1—O-A1-O-A2-(O-A3)n—O—R2 (wherein R1 and R2 respectively represent a C1-C3 alkyl group; A1, A2 and A3 respectively represent an ethylene group or a propylene group; and n represents 0, 1 or 2), and not less than 12% by weight but not more than 90% by weight of 1,3-dimethyl-2-imidazolidinone. The emulsifiable concentrate composition is also characterized in that the weight ratio of 1,3-dimethyl-2-imidazolidinone to the ether compound (1,3-dimethyl-2-imidazolidinone:ether compound) is within the range from 1:0.03 to 1:2.
Owner:SUMITOMO CHEM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com