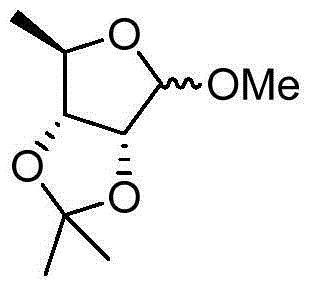
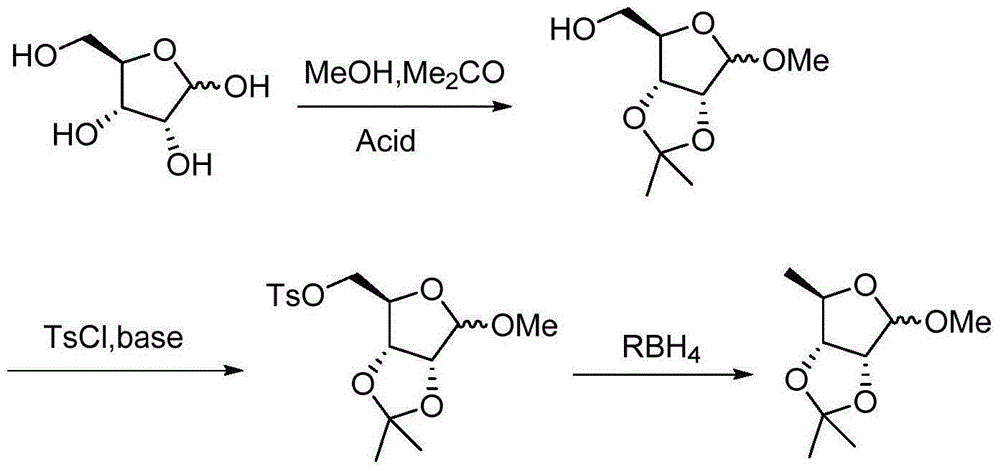
Preparation method of methyl-2,3-O-isopropylidene-5-deoxy-D-ribofuranoside
A ribofuranoside and isopropylidene technology is applied in the field of methyl-2,3-O-isopropylidene-5-deoxy-D-ribofuranoside preparation, and can solve the problem of complicated operation, difficulty in recycling and recovery Difficulties and other problems, to achieve the effect of mild conditions and low production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add 50g of D-ribose, 400ml of methanol, 400ml of acetone, and 4g of methanesulfonic acid into a 1000ml three-necked flask, stir to dissolve, heat up to reflux for 2.5 hours, cool to room temperature, add triethylamine to adjust the pH to 7-8, and concentrate under reduced pressure to remove Methanol and acetone, cooled to 15-20°C, diluted with 50ml of water, extracted with 300ml+200ml of toluene. Combine the toluene phases, cool to 5-10°C, add 57.5g of p-toluenesulfonyl chloride, 1g of triethylbenzyl ammonium chloride, dropwise add 54g of 50% sodium hydroxide solution, keep the reaction for 4 hours after dropping, add 5ml of methanol, continue React for 1 hour, add 100ml×2 water to wash the salt, add 200ml saturated aqueous sodium bicarbonate solution to the toluene phase, heat up and reflux for 1 hour, cool to room temperature, and separate the phases to obtain methyl-2,3-O-isopropylidene-5- O-toluenesulfonyl-D-ribofuranoside toluene is about 550ml.
Embodiment 2
[0022] The methyl-2,3-O-isopropylidene-5-O-p-toluenesulfonyl-D-ribofuranoside toluene obtained in Example 1 was mixed with about 550ml of toluene, concentrated under reduced pressure to remove the toluene, added 200ml of HMPA, and heated to 90-95 ℃, slowly add 16.2g of potassium borohydride in batches, keep warm for 4 hours after addition, cool to 15-20℃, add 200ml of water dropwise to quench the reaction. Extract with 150ml×3 petroleum ether, combine the petroleum ether phases, wash with 20ml of water, concentrate to remove the petroleum ether, and obtain 50g of methyl-2,3-O-isopropylidene-5-deoxy-D-ribofuranoside, The total yield is 80%, and the GC content is 96.9%.
Embodiment 3
[0024] The methyl-2,3-O-isopropylidene-5-O-p-toluenesulfonyl-D-ribofuranoside toluene obtained in Example 1 contained about 550ml of toluene, concentrated under reduced pressure to remove the toluene, added 200ml of DMI, and raised the temperature to 90-95 ℃, slowly add 16.2g of potassium borohydride in batches, keep warm for 4 hours after addition, cool to 15-20℃, add 200ml of water dropwise to quench the reaction. Extract with 150ml×3 petroleum ether, combine the petroleum ether phases, wash with 20ml of water, concentrate to remove the petroleum ether, and obtain 51g of methyl-2,3-O-isopropylidene-5-deoxy-D-ribofuranoside, The total yield is 81%, and the GC content is 97.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com