Method for preparing 5-nitro-2-furaldehyde and nifuratel
A technology of nitrofurfural and nitro group is applied in the field of preparation of 5-nitrofurfural and nifuratel, and can solve the problems of low yield and purity of 5-nitrofurfural, low total yield of nifuratel and easy Problems such as the formation of polymers, to achieve the effect of less impurities, shortened reaction time, and reduced side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] (1) Preparation of 5-nitrofurfural (compound 2)
[0050]
[0051] In a 100L reactor, add ethanol 60L, 5-nitrofurfural diethyl ester (compound 1) 20kg (82.25mol), 10% sulfuric acid (m / v) 1.5L, indium trichloride 910.06g (4.11mol), Heated to 80-85°C, reacted for 1.5h, concentrated under reduced pressure to remove ethanol to obtain a concentrate; the concentrate was dissolved in dichloromethane, washed three times with saturated sodium chloride, and the dichloromethane layer was collected; Dry over sodium sulfate, filter to remove the solid sodium sulfate, concentrate under reduced pressure to obtain the crude product of 5-nitrofurfural; the crude product is purified by silica gel column chromatography (eluent PE:EA=5:1), collect the target substance, and concentrate under reduced pressure , to obtain pure 5-nitrofurfural with a purity of 98% and a yield of 82%.
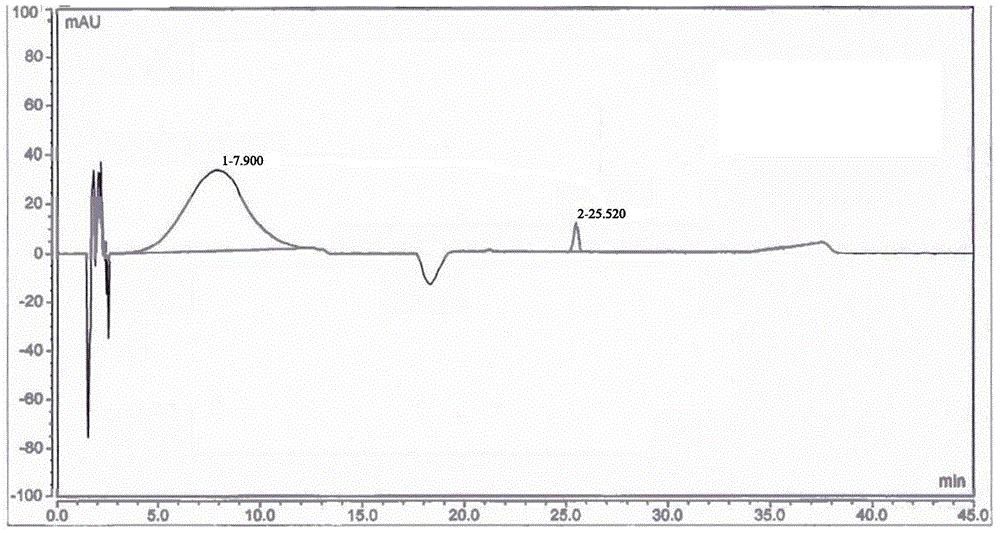
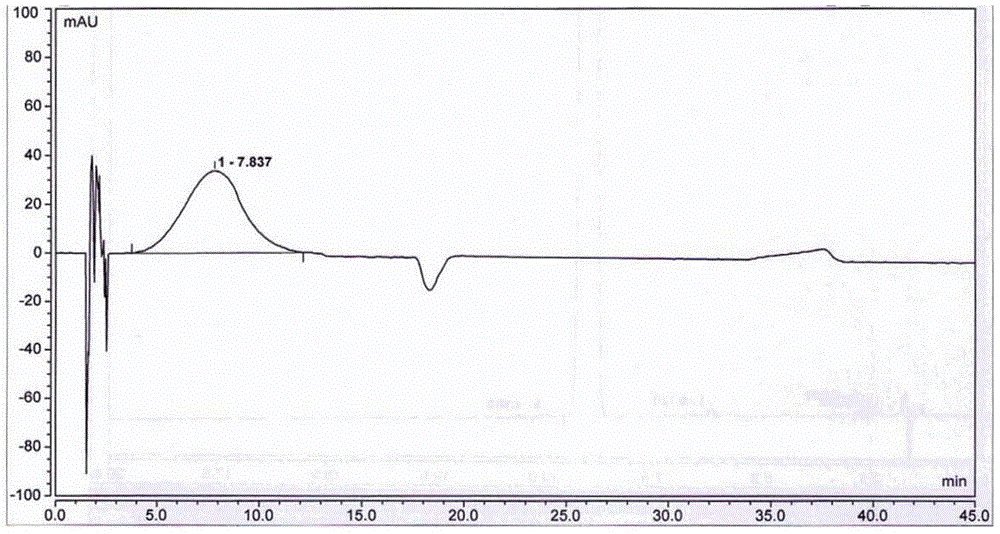
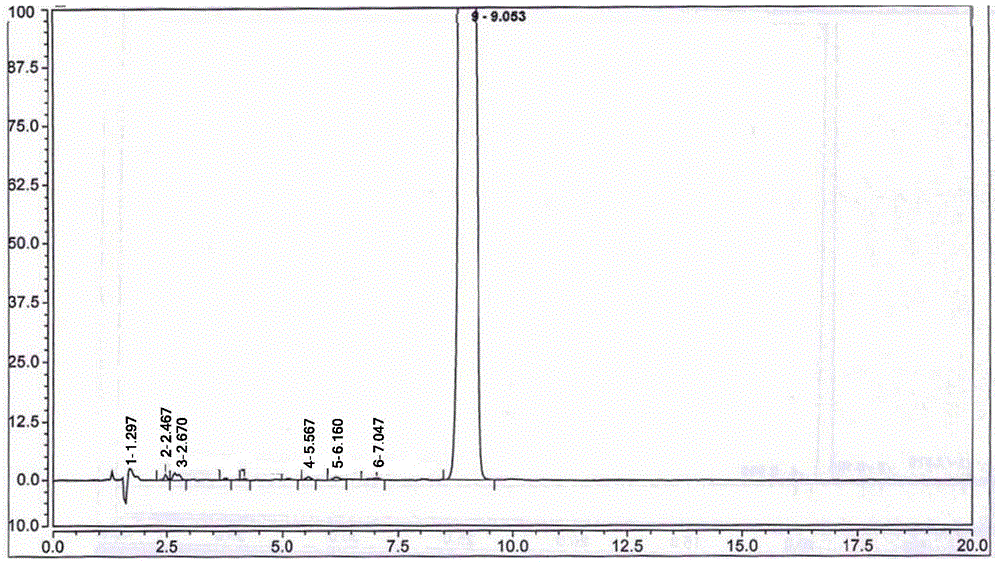
[0052] Adopt high performance liquid chromatography (HPLC) to detect the 5-nitrofurfural pure product that...
Embodiment 2
[0067] (1) Preparation of 5-nitrofurfural (compound 2)
[0068] In a 100L reactor, add ethanol 60L, 5-nitrofurfural diethyl ester (compound 1) 20kg (82.25mol), 10% sulfuric acid (m / v) 1.5L, InCl 3 / HC(OEt) 3 (indium trichloride 4.11mol, InCl 3 with HC(OEt) 3 The molar ratio is 1:0.5), heated to 78-85°C, reacted for 1h, concentrated under reduced pressure to remove ethanol, and obtained the concentrate; the concentrate was dissolved in dichloromethane, washed three times with saturated sodium chloride, and the dichloromethane was collected layer; the dichloromethane layer was dried with anhydrous sodium sulfate, filtered to remove the sodium sulfate solid, and concentrated under reduced pressure to obtain the crude product of 5-nitrofurfural; the crude product was purified by silica gel column chromatography (eluent PE:EA=5:1) , collect the target substance, and concentrate under reduced pressure to obtain the pure product of 5-nitrofurfural with a yield of 69.3%.
[0069] ...
Embodiment 3
[0075] (1) Preparation of 5-nitrofurfural (compound 2)
[0076] In a 100L reactor, add ethanol 60L, 5-nitrofurfural diethyl ester (compound 1) 20kg (82.25mol), 10% sulfuric acid (m / v) 1.5L, InCl 3 / HC(OEt) 3 (4.11mol, InCl 3 with HC(OEt) 3 The molar ratio is 1:1.0), heated to 78-85°C, reacted for 1.5h, concentrated under reduced pressure to remove ethanol to obtain a concentrate; the concentrate was dissolved in dichloromethane, washed three times with saturated sodium chloride, and collected dichloro The methane layer; the dichloromethane layer was dried with anhydrous sodium sulfate, filtered to remove the sodium sulfate solid, and concentrated under reduced pressure to obtain the crude product of 5-nitrofurfural; the crude product was purified by silica gel column chromatography (eluent PE:EA=5:1 ), collect the target substance, and concentrate under reduced pressure to obtain the pure product of 5-nitrofurfural with a yield of 89.2%.
[0077] If nifuratel is prepared, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com