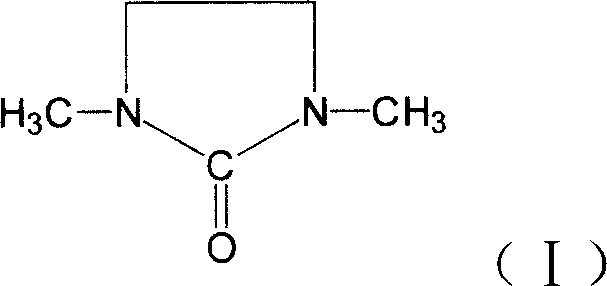
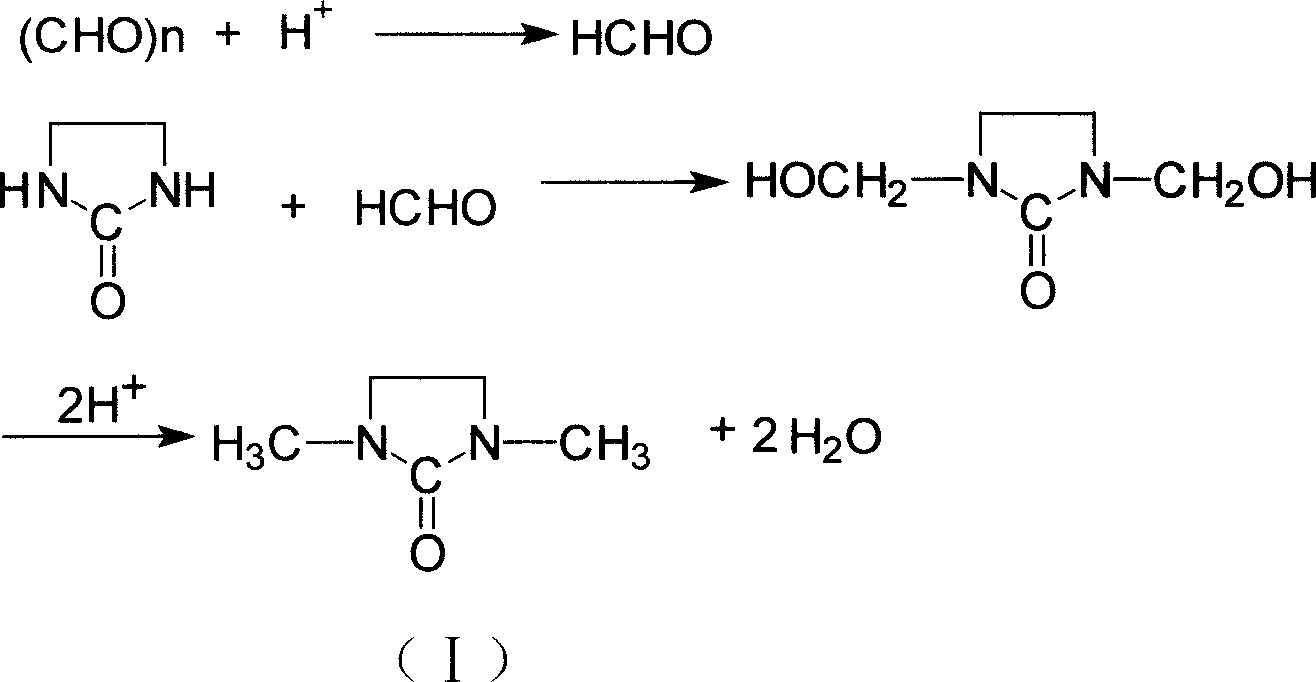
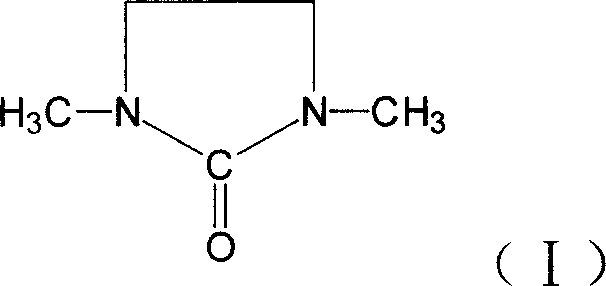
Synthesizing method for 1,3, dimethyl-2-imidazolidinone
A technology of imidazolinone and synthesis method, applied in 1 field, can solve problems such as inability to use, and achieve the effects of improving utilization rate, easy control of reaction conditions, and reducing material cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 501g (3.626mol) 33.3% formic acid, 107g paraformaldehyde (3.567mol based on formaldehyde) and 153g (1.779mol) 2-imidazoline to a 1000mL four-day bottle equipped with stirring, thermometer, and reflux condenser Ketone, stirred, heated to 70°C, reacted for 4 hours, then added 237g (4.379mol) 85% formic acid, heated and refluxed for 12 hours, and distilled out the formic acid. The reactant was neutralized with sodium hydroxide solution, vacuum distilled, and the 105°C-118°C fraction was collected at a vacuum degree of 0.095Mpa to obtain 1,3-dimethyl-2-imidazolidinone with a purity of 99.7% by gas chromatography. Yield 78%.
[0030] bp=225.5°C
[0031] IR (KBr cm -1 ) 1249 1290, 1504, 1699, 2862, 2938
example 2
[0033] In a 1000mL four-necked flask equipped with stirring, a thermometer, and a reflux condenser, add 475g (4.234mol) of 41.8% formic acid, 117g of paraformaldehyde (calculated as 3.900mol by formaldehyde), 153g (1.779mol) of 2-imidazoline Ketone, stirred, heated to 80°C, reacted for 3 hours, then added 195g (3.985mol) 94% formic acid, heated to reflux for 12 hours, evaporated 413g of formic acid, the concentration of formic acid was 45%. The reactant was neutralized with sodium hydroxide solution, vacuum distilled, and the 105°C-118°C fraction was collected at a vacuum degree of 0.095Mpa to obtain 1,3-dimethyl-2-imidazolidinone with a purity of 99.9% by gas chromatography. Yield 79.2%.
[0034] bp=225.5°C
[0035] IR (KBr cm -1 ) 1249 1290, 1504, 1699, 2862, 2938
example 3
[0037] In the 1000mL four-neck flask equipped with stirring, thermometer, and reflux condenser, add 170g paraformaldehyde (calculated as 5.667mol by formaldehyde), the formic acid 413g (4.040mol) of example 2 reclaims 45% formic acid, 153g (1.779mol ) 2-imidazolinone, stirred, heated to 90°C, reacted for 2 hours, then added 216g (3.985mol) 85% formic acid, heated to reflux for 12 hours, distilled 467g of dilute formic acid, the concentration was 41%. The reactant was neutralized by adding sodium hydroxide solution, vacuum distilled, and the 105°C-118°C fraction was collected at a vacuum degree of 0.095Mpa to obtain 1,3-dimethyl-2-imidazolidinone, whose purity by gas chromatography analysis was 99.93%. Yield 81.3%.
[0038] bp=225.5°C
[0039] IR (KBr cm -1 ) 1249 1290, 1504, 1699, 2862, 2938.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com