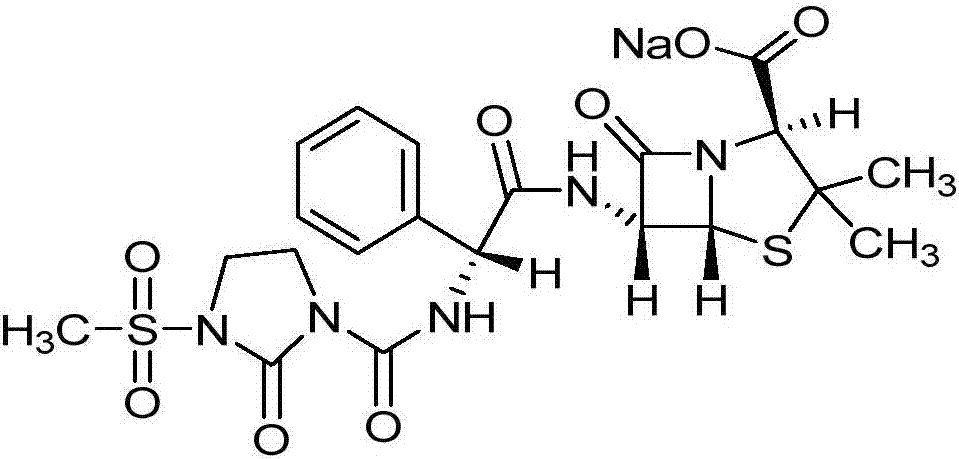
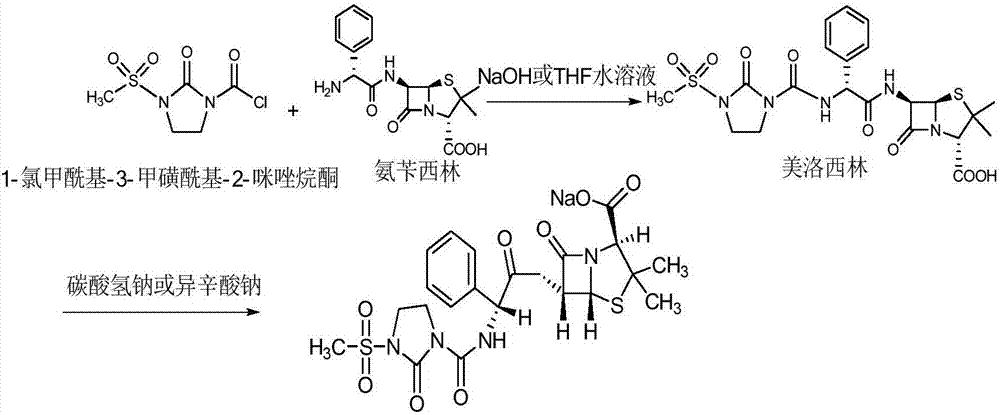
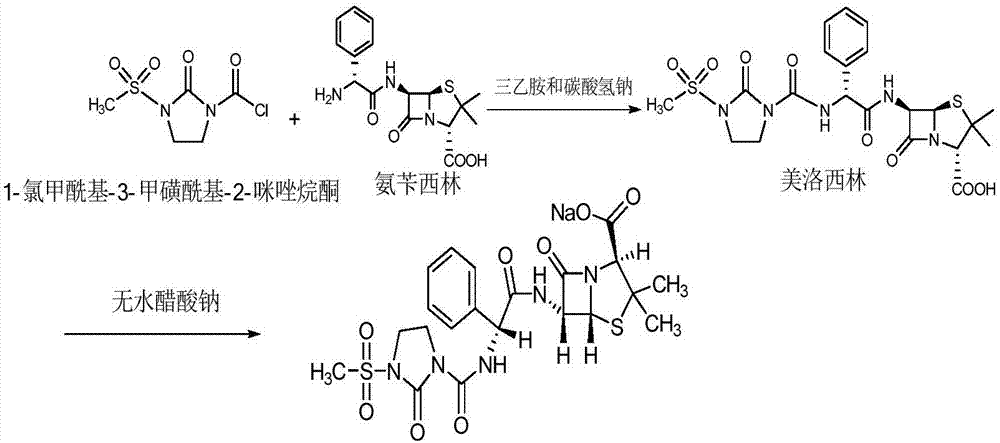
Preparation technology of mezlocillin sodium
A mezlocillin sodium and preparation technology, applied in the direction of organic chemistry, etc., can solve the problems of the large gap between original drugs and the large amount of impurities in mezlocillin sodium, and achieve the effect of reducing impurities and reducing side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The preparation technology of mezlocillin sodium comprises the following steps:
[0028] (1) Add 180mL of purified water to a 1000mL three-neck flask, control the temperature at 5°C, add 20g of ampicillin trihydrate, add triethylamine to adjust the pH to 9.5, dissolve it, add 6.0g of 1-chloroformyl-3- The mixture of methylsulfonyl-2-imidazolidinone and 100mL ethyl acetate was controlled at a temperature of 5°C, and the dropwise addition time was 30min, 6.0g of sodium bicarbonate was added, stirred for 15min, and then 6.0g of 1-chloroformyl - Add the mixture of 3-methylsulfonyl-2-imidazolidinone and 100mL ethyl acetate dropwise to the above solution for 120min, control the pH to 7.2, and react for 30min after the dropwise addition, add 1g of activated carbon to the feed solution to decolorize 30min, temperature 5°C, filter;
[0029] (2) Raise the temperature of the feed solution to 19°C, add hydrochloric acid with a volume concentration of 4mol / L to acidify to pH 1.2, l...
Embodiment 2
[0031] The preparation technology of mezlocillin sodium comprises the following steps:
[0032] (1) Add 180mL of purified water to a 1000mL three-neck flask, control the temperature at 2°C, add 20g of ampicillin trihydrate, add triethylamine to adjust the pH to 9.0, dissolve it, add 6.0g of 1-chloroformyl-3- The mixture of methylsulfonyl-2-imidazolidinone and 100mL ethyl acetate was controlled at a temperature of 2°C, and the dropwise addition time was 30min. Added 5.5g of sodium bicarbonate, stirred for 15min, and then added 6.5g of 1-chloroformyl - Add the mixture of 3-methylsulfonyl-2-imidazolidinone and 100mL ethyl acetate dropwise to the above solution for 120min, control the pH to 6.8, and react for 30min after the dropwise addition, add 1g of activated carbon to the feed solution to decolorize 30min, temperature 2°C, filter;
[0033] (2) Raise the temperature of the feed solution to 16°C, add hydrochloric acid with a volume concentration of 4mol / L to acidify to a pH of...
Embodiment 3
[0035] The preparation technology of mezlocillin sodium comprises the following steps:
[0036] (1) Add 180mL of purified water to a 1000mL three-neck flask, control the temperature at 4°C, add 20g of ampicillin trihydrate, add triethylamine to adjust the pH to 8.7, dissolve it, add 6.3g of 1-chloroformyl-3- The mixture of methylsulfonyl-2-imidazolidinone and 100mL ethyl acetate was controlled at a temperature of 4°C, and the dropwise addition time was 40min. Added 5.0g of sodium bicarbonate, stirred for 15min, and then added 6.7g of 1-chloroformyl - Add the mixture of 3-methylsulfonyl-2-imidazolidinone and 100mL ethyl acetate dropwise to the above solution for 100min, control the pH to 7.0, and react for 30min after the dropwise addition, add 1g of activated carbon to the feed solution for decolorization 30min, temperature 4°C, filter;
[0037] (2) Raise the temperature of the feed solution to 18°C, add hydrochloric acid with a volume concentration of 4mol / L to acidify to pH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com