Polyamic acid ester liquid crystal alignment agent, and liquid crystal alignment film using same
A technology of liquid crystal aligning agent and polyamic acid ester, which is applied in coatings, instruments, optics, etc., can solve the problems of unimproved properties of polyimide liquid crystal aligning agents, high volume resistivity, and high residual charge. Achieve the effect of improving reliability, improving electrical characteristics, and improving interface characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1)A-5
[0362] (Synthesis Example 1) Synthesis of A-5
[0363] The diamine compound (A-5) was synthesized through the four-step route shown below.
[0364] Step 1: Synthesis of compound (A5)
[0365] [Chemical 58]
[0366]
[0367] Add propargylamine (8.81g, 160mmol), N,N-dimethylformamide (112mL), and potassium carbonate (18.5g, 134mmol) in sequence in a 500mL eggplant-shaped flask to make it 0°C, and use A solution obtained by dissolving tert-butyl bromoacetate (21.9 g, 112 mmol) in N,N-dimethylformamide (80 mL) was added dropwise with stirring for about 1 hour. After completion of the dropwise addition, the reaction solution was brought to room temperature and stirred for 20 hours. Then, the solid content was removed by filtration, 1 L of ethyl acetate was added to the filtrate, and the mixture was washed four times with 300 mL of water and once with 300 mL of saturated brine. Then, the organic layer was dried over magnesium sulfate, and the solvent was distilled off under r...
Synthetic example 2)A-2
[0380] (Synthesis Example 2) Synthesis of A-2
[0381] The diamine compound (A-2) was synthesized by the 2-step route shown below.
[0382] Step 1: Synthesis of compound (A8)
[0383] [Chemical 61]
[0384]
[0385] Add 2-iodo-4-nitroaniline (5.11g, 19.4mmol), bis(triphenylphosphine)palladium(II) dichloride (281.7mg, 0.401mmol) into a four-necked flask replaced with nitrogen, iodine Copper chloride (160.7mg, 0.844mmol) and 30ml of diethylamine were stirred at room temperature (20°C) for 10 minutes. Then, t-butyl N-propargylglycine (compound (5)) (3.72 g, 24.0 mmol) was added, and the mixture was stirred at room temperature (20° C.) for 4 hours. After confirming the disappearance of the raw materials by HPLC, 200 ml of ethyl acetate and 200 ml of a 1M ammonium chloride aqueous solution were added for extraction. The obtained organic layer was washed twice with a 1M aqueous ammonium chloride solution, and dried over anhydrous magnesium sulfate. After removing the desicca...
Synthetic example 3
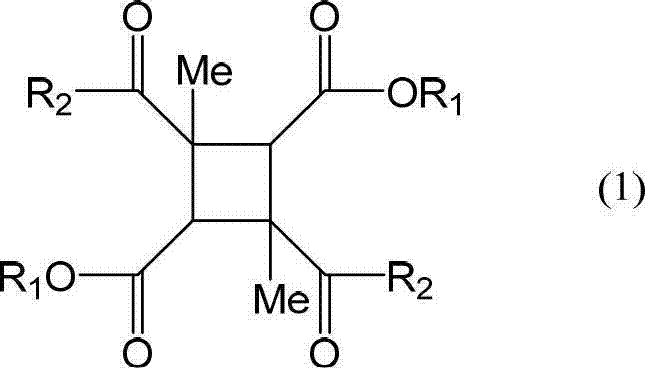
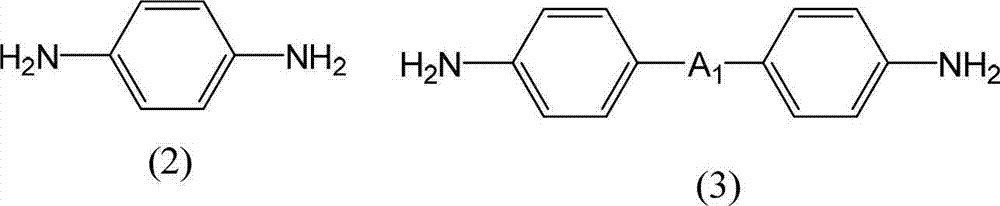
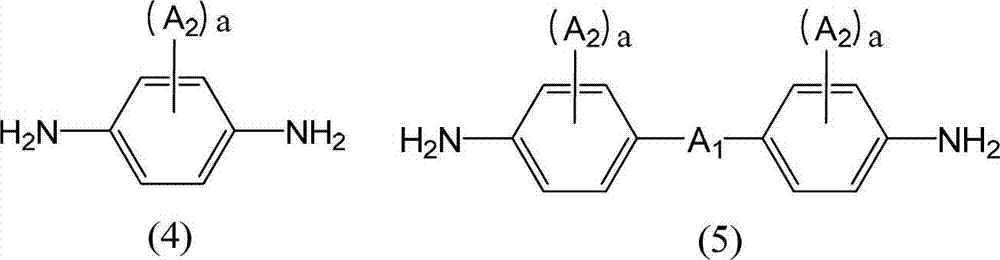
[0390]Make a 3L four-necked flask with a stirring device a nitrogen atmosphere, add 44.04g (0.407mol) of p-phenylenediamine, 43.59g (0.115mol) of diamine (A-5), add NMP 1891g and pyridine as a base 92.19g (1.17mol), stirred to dissolve. Next, stirring this diamine solution, 157.90 g (0.486 mol) of 1,3DM-CBDE-Cl was added, and it was made to react under water cooling for 4 hours. 2101 g of NMP was added to the obtained polyamic acid ester solution, it stirred for 30 minutes, and the polyamic acid ester solution which can obtain 5 weight% of solid content was obtained. The obtained polyamic acid ester solution was added to 21012g of water while being stirred, and the precipitated white precipitate was filtered out, followed by washing once with 21012g of water, once with 21012g of ethanol, and three times with 5253g of ethanol. After drying, 175.94 g of white polyamic acid ester resin powder was obtained. The yield was 83.7%. Moreover, the molecular weight of this polyamic ac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com