Patents
Literature
87 results about "Diethylene glycol diethyl ether" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
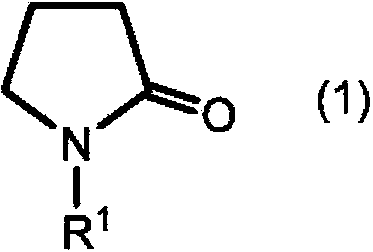
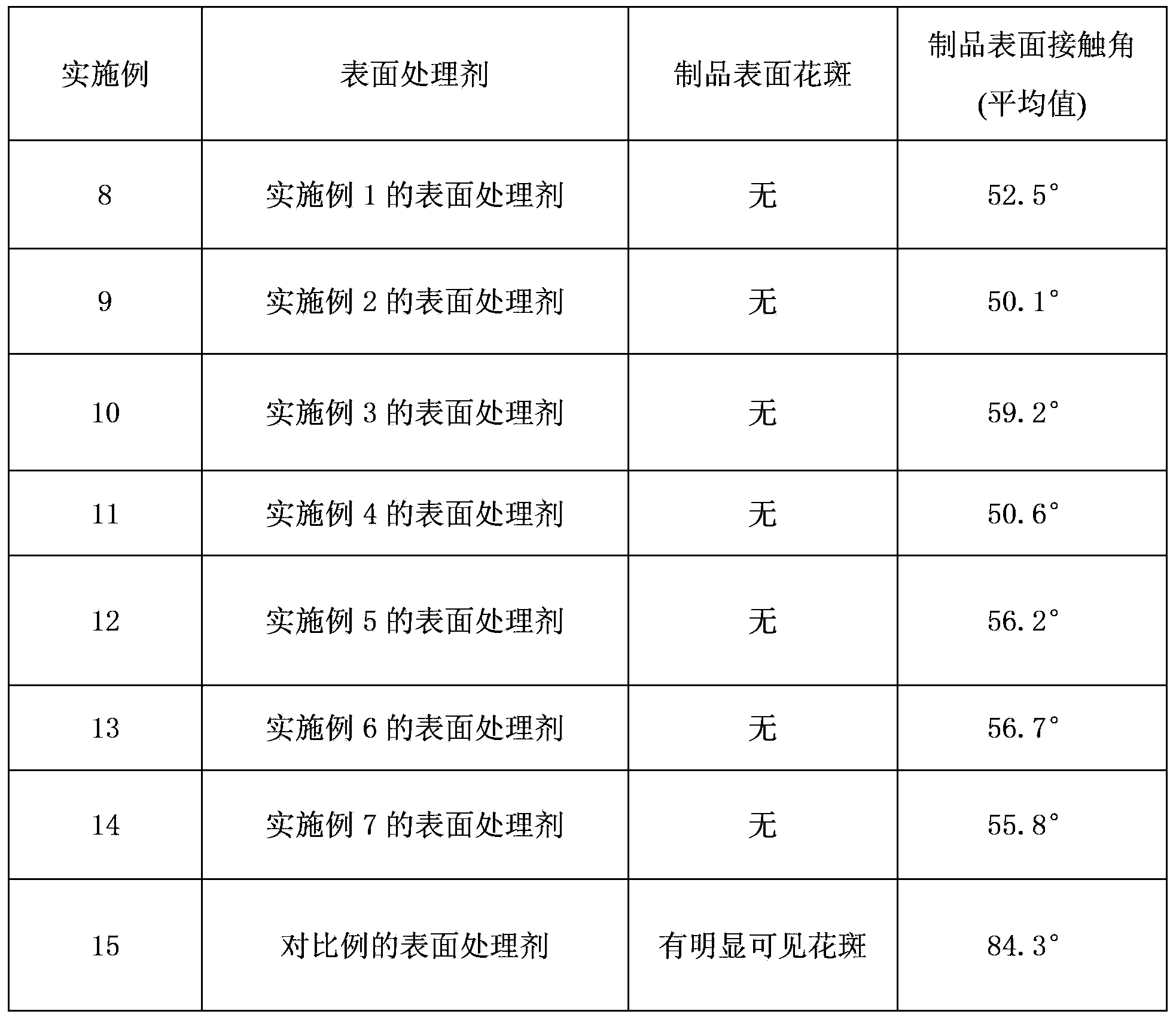
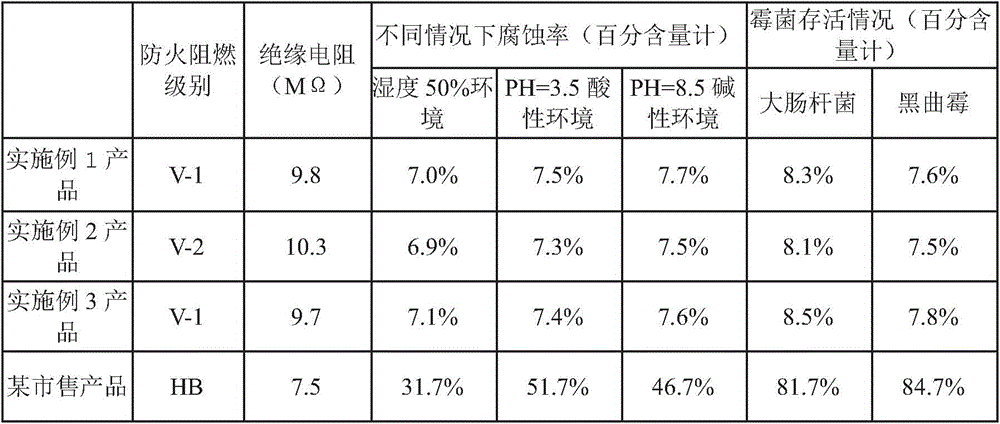
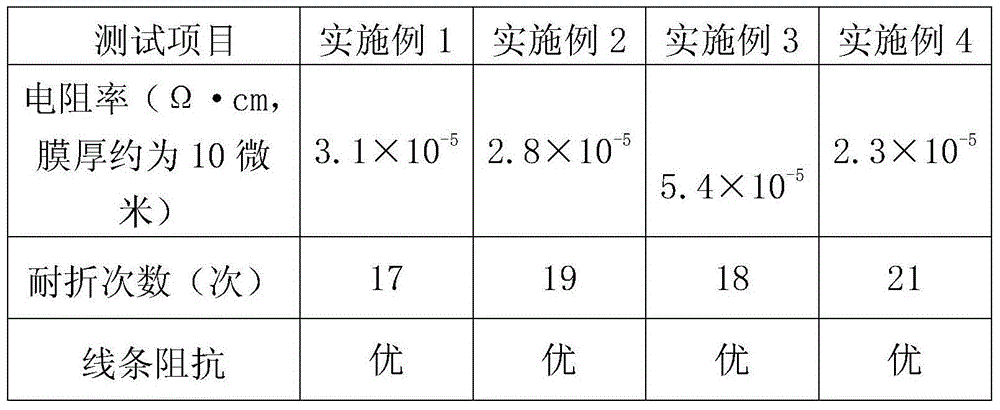
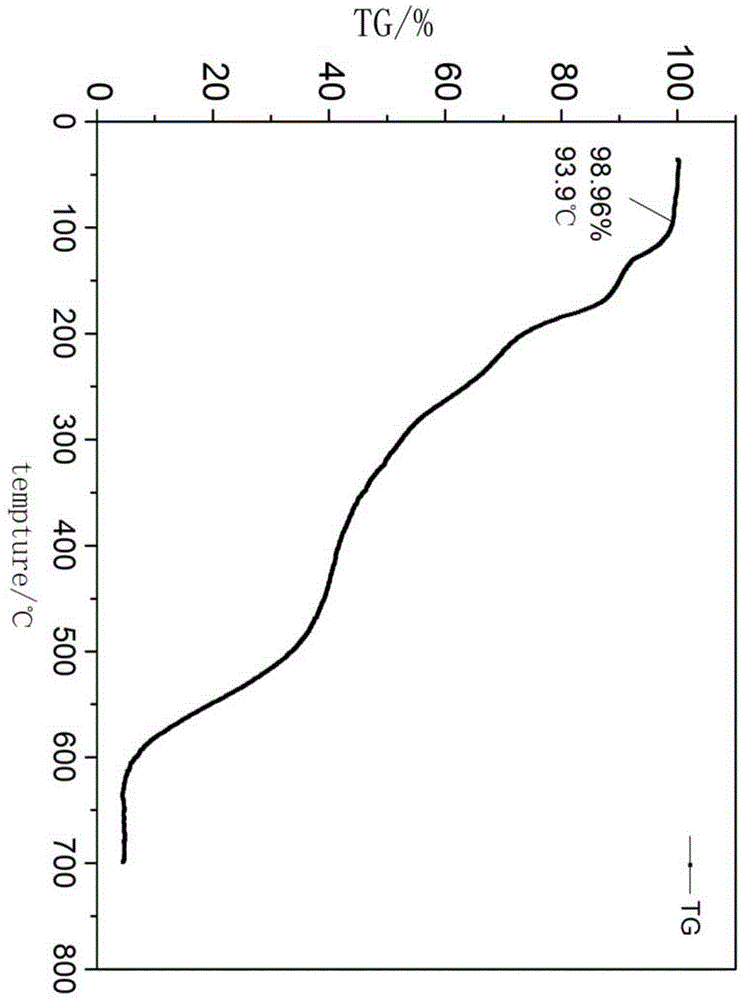
Diethylene glycol diethyl ether is an organic solvent with a high boiling point.
Aqueous film-forming foam extinguishing agent special for fire extinguishing bullet and throwing type fire extinguisher
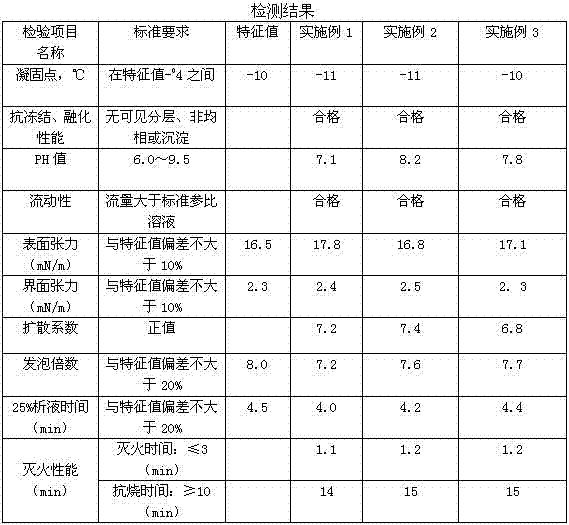
InactiveCN104117176AImprove fire extinguishing effectPrevent re-ignitionFire extinguisherDiethylene glycol diethyl etherAmmonium dodecyl sulfate
The invention provides an aqueous film-forming foam extinguishing agent special for a fire extinguishing bullet and a throwing type fire extinguisher. The aqueous film-forming foam extinguishing agent has the advantages of being small in additive amount, rapid in fire extinguishing, good in recrudescence resisting effect, environmentally friendly, efficient and the like. The aqueous film-forming foam extinguishing agent comprises, by weight, 8 percent to 12 percent of main foaming agents, 8 percent to 20 percent of auxiliary foaming agents, two percent to 10 percent of antifreeze agents, 12 percent to 22 percent of cosolvents, 0.1 percent to one percent of stabilizers, 8 percent to 20 percent of fire retardants, two percent to five percent of burning-resistance agents and the balance water. The main foaming agents are selected from F1157N, F1203 and F1460, the auxiliary foaming agents are selected from lauryl sodium sulfate, ammonium lauryl sulfate, alkyl glycoside, dodecyl dimethyl betaine and cocamidopropyl betaine. The antifreeze agents are selected from ethylene glycol, glycerol and the like. The cosolvents are selected from dodecanol, n-butyl alcohol and diethylene glycol diethyl ether. The stabilizing agents are selected from xanthan gum, Arabic gum, guar gum and sodium alginate tech grade. The fire retardants are selected from ammonium polyphosphate and ammonium dihydrogen phosphate.
Owner:ANHUI TIANYUAN FIRE PROTECTION TECH
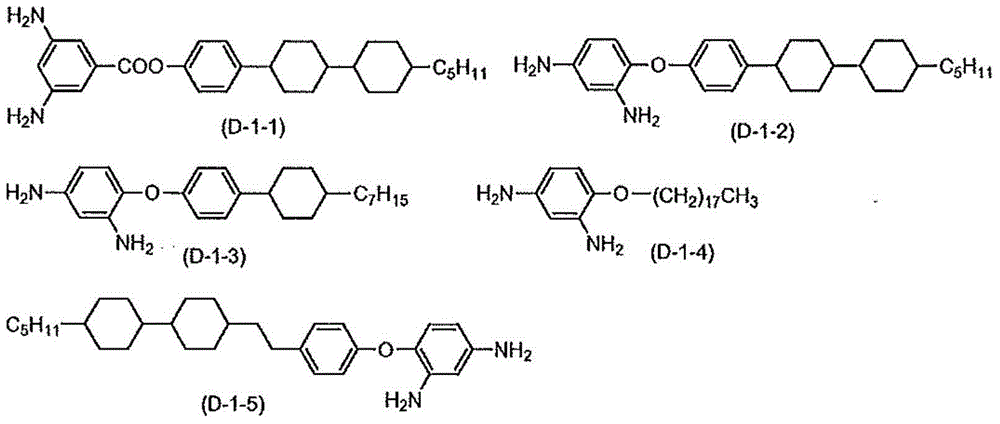
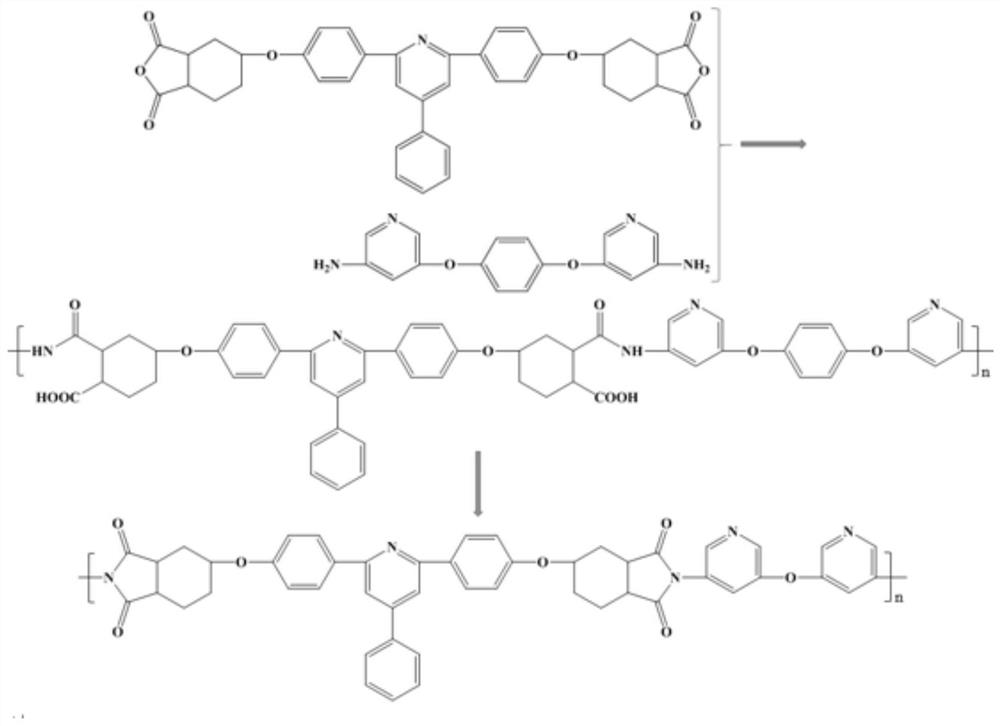
Aligning agent for liquid crystal and liquid-crystal alignment film obtained with the same
ActiveUS20070036915A1Good film uniformityLiquid crystal compositionsThin material handlingCrystallographyDiethylene glycol diethyl ether
To provide a liquid crystal aligning agent which gives a coating film with good uniformity regardless of drying temperature after coating, and a liquid crystal alignment film having good coating film uniformity. A liquid crystal aligning agent comprising at least one polymer selected from a polyamic acid and a soluble polyimide, diethylene glycol diethyl ether, and dipropylene glycol monomethyl ether, and a liquid crystal alignment film obtained by printing this liquid crystal aligning agent by a flexographic printing method.
Owner:NISSAN CHEM IND LTD
Composition for forming a liquid crystal alignment film, and liquid crystal display device
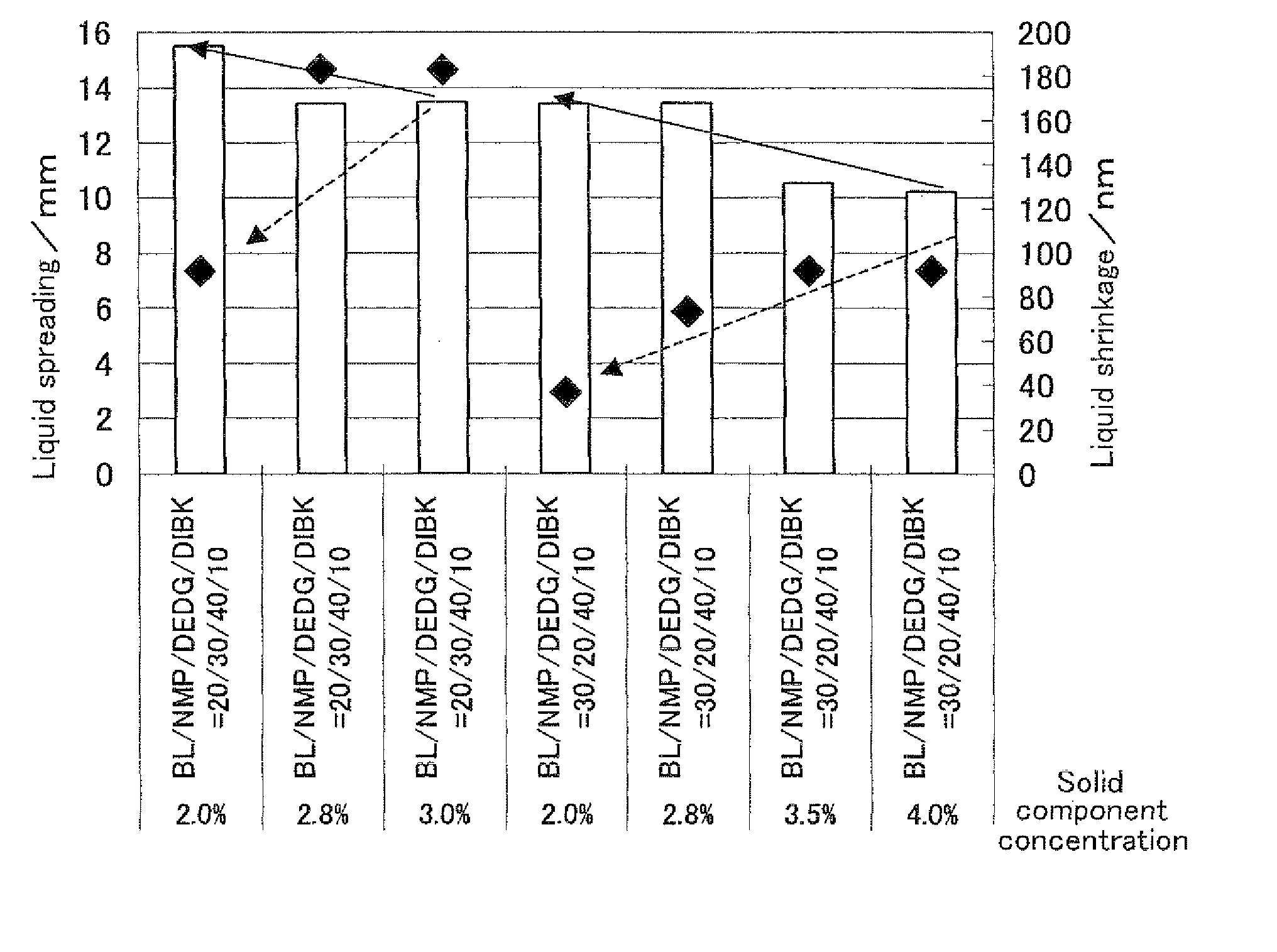
ActiveUS20110007254A1Good coating performanceSuppress display unevennessLiquid crystal compositionsPlastic/resin/waxes insulatorsDiethylene glycol diethyl etherLiquid-crystal display
The present invention provides: a composition for forming a liquid crystal alignment film capable of forming a liquid crystal alignment film excellent in evenness; and a liquid crystal display device. The present invention provides a composition for forming a liquid crystal alignment film, wherein the composition comprises: a material for forming a liquid crystal alignment film; diethylene glycol diethyl ether; diisobutyl ketone; and at least one of γ-butyrolactone and N-methyl-2-pyrrolidone as solvents.
Owner:SHARP KK +1
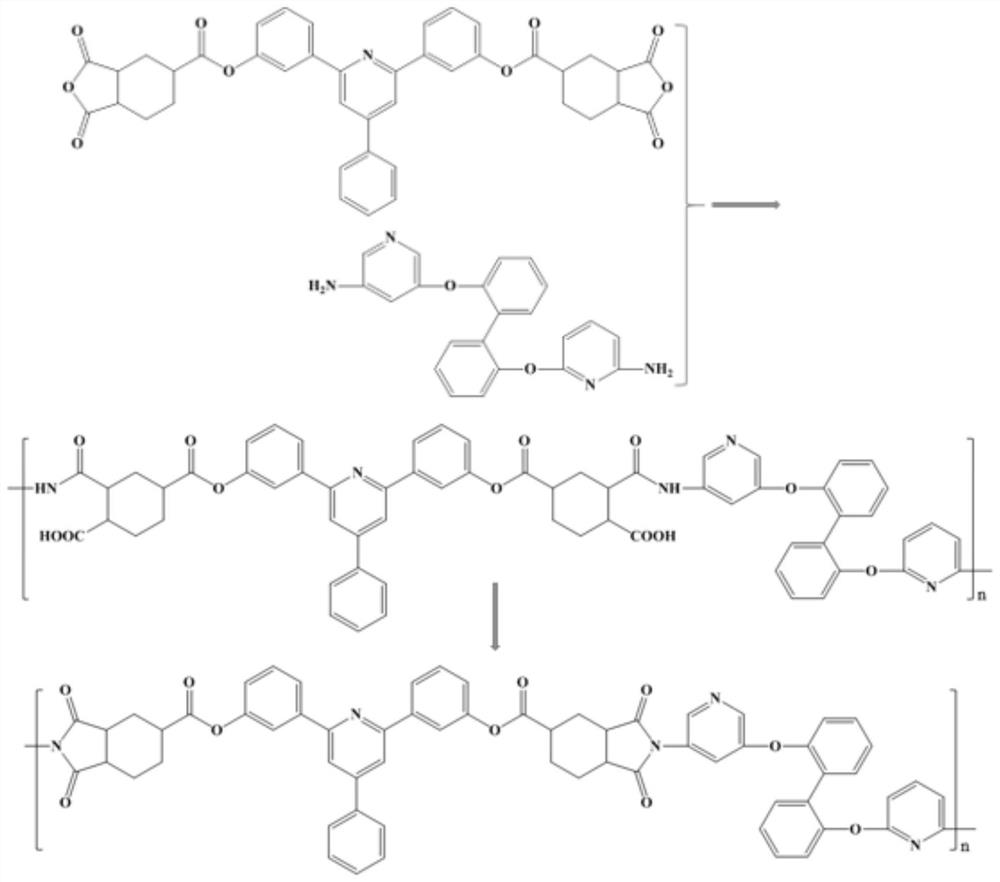
Liquid crystal alignment agents for forming horizontal alignment film and liquid crystal display element
The invention provides a liquid crystal oriented agent used for forming a horizontal alignment film, and a liquid crystal display element. Wherein, the liquid crystal oriented agent comprises polymer selected from at least one of polyamide acid and imide polymer of the polyamide acid and mixed solvent. The mixed solvent comprises: a first solvent selected from at least one of N-methyl-2-pyrrolidon, gamma-butyrolactone, 1,3-dimethyl-2-imidazolinone, N,N-dimethylformamide and N,N-dimethylacetamide; a second solvent selected from at least one of Butyl Cellosolve, diacetone alcohol, propylene carbonate, diethylene glycol diethyl ether and ethide-3-ethoxypropionate; a third solvent expressed by the following formula (A) and with the weight rate relative to the total solvent weight in the range of 0.1wt% to 15wt%. The formula is R<1>-Z-R<2> (A). R<1> and R<2> are alkyl. Z is -COO-, -O- or -CO-. Wherein, when Z is -COO-, carbon number of R<1> and R<2> totaled in the range of 6 to 9, and when Z is -O-, the carbon number of R<1> and R<2> totaled in the range of 6-10, and when Z is -CO-, the carbon number of R<1> and R<2> totaled 8.
Owner:JSR CORPORATIOON
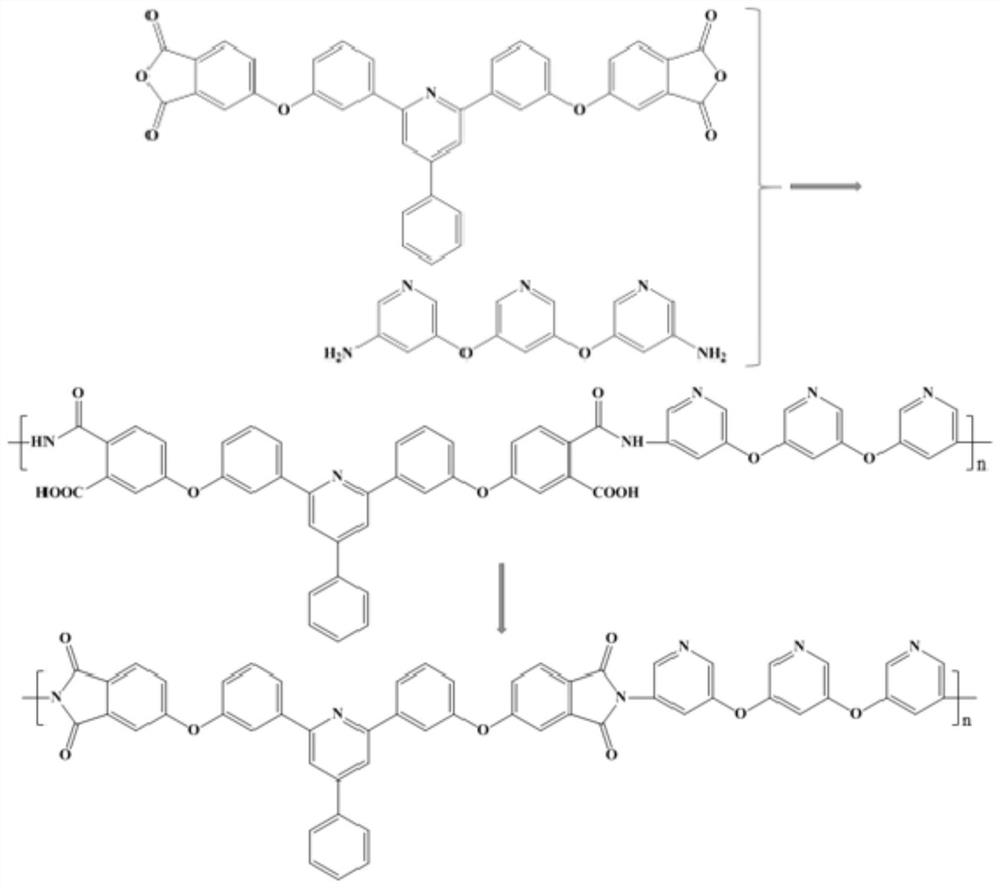
Liquid crystal orientation agent, liquid crystal orientation film, manufacturing method of liquid crystal orientation film, and liquid crystal display element
InactiveCN103666486AImprove yieldUniform coatingLiquid crystal compositionsNon-linear opticsDiacetone alcoholDiethylene glycol diethyl ether
The invention relates to a liquid crystal orientation agent, a liquid crystal orientation film, a manufacturing method of the liquid crystal orientation film, and a liquid crystal display element. The liquid crystal orientation agent has good printing performance with respect to a substrate and good continuous printing performance and prevents swelling of printing plates. The liquid crystal orientation agent contains at last one polymer (A) selected from the group formed by polyamide acid, polyimide, polyamide acid ester and polysiloxane, a first solvent containing more than one of N-ethyl-2-pyrrolidone, 1,3-dimethyl-2-imidazolone, 3-methoxy-N, N-dimethylpropionamide and so on, and a second solvent containing more than one of propylene glycol diacetate, diethylene glycol diethyl ether, diisoamyl ether diacetone alcohol and so on.
Owner:JSR CORPORATIOON
Scaling powder for low-temperature halogen-free low-solid-content lead-free tin soldering
InactiveCN103785973AEnhanced spread rate effectHigh spreading rateWelding/cutting media/materialsSoldering mediaDiethylene glycol diethyl etherSalicylic acid
The invention discloses scaling powder for low-temperature halogen-free low-solid-content lead-free tin soldering. The scaling powder comprises 12 percent to 16 percent of activating agents, 8-10 percent of colophony, 2-3 percent of surface active agents, 0.05-0.1 percent of antioxidants, 5-8 percent of organic amine and the balance organic solvents. The activating agents comprise compound components of 40-57 parts of anhydrous citric acid, 7-14 parts of salicylic acid, 0.6-3.6 parts of lactic acid and 36-43 parts of DL-malic acid, the surface active agents comprise compound component Tween 60 and a Span, the organic amine is one or more of monoethanolamine, diethanol amine and triethanolamine, the antioxidants are tertiary butylhydroquinone, and the organic solvents are mixed solvents of ethylene glycol, diethylene glycol diethyl ether, nitroethane, tetrahydrofurfuryl alcohol and propylene glycol by mass. According to the method, the scaling powder for low-temperature halogen-free low-solid-content lead-free tin soldering has the advantages of improving the coverage rate of the welding materials and reducing the cost.
Owner:SUZHOU LOTTE CHEM TECH
Liquid crystal aligning agent, liquid crystal aligning film and liquid crystal display element
ActiveCN105001881AReduce non-display areaAchieve narrow frameLiquid crystal compositionsNon-linear opticsDiethylene glycol diethyl etherDiacetone alcohol
The invention provides a liquid crystal aligning agent, a liquid crystal aligning film and a liquid crystal display element. The liquid crystal aligning agent contains at least one polymer (A) selected from the group consisting of polyamic acid, polyamic acid ester and polyimide, and solvents. The solvents contain a specific solvent (X) and a specific solvent (Y). The specific solvent (X) is 1-butoxy-2-propanol; and the specific solvent (Y) contains at least one of the group consisting of diethylene glycol diethyl ether, diacetone alcohol, propylene glycol diacetate and dipropylene glycol monomethyl ether. According to the invention, undesirable film thickness of the end of the coating area of the liquid crystal aligning agent is limited.
Owner:JSR CORPORATIOON
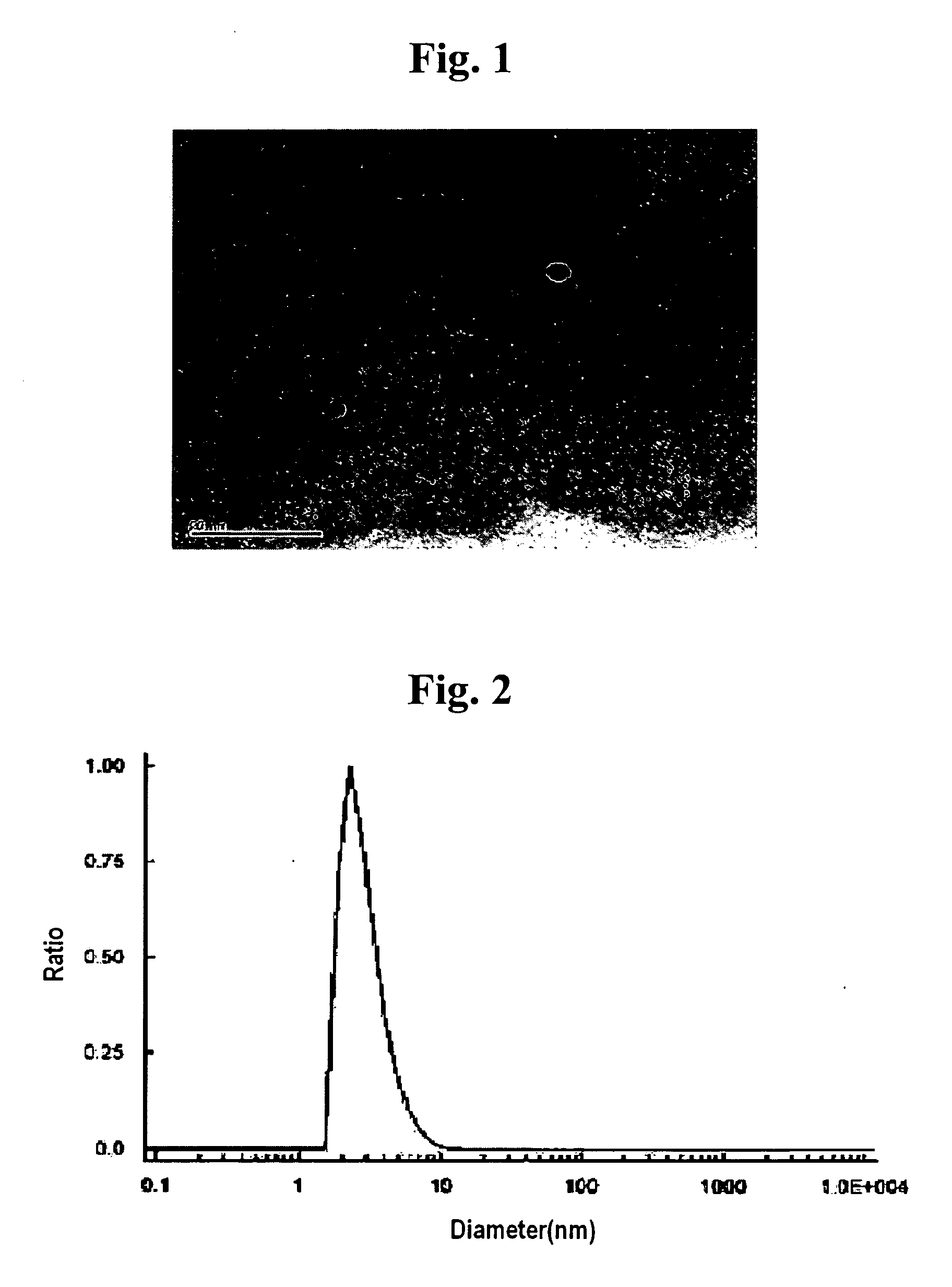
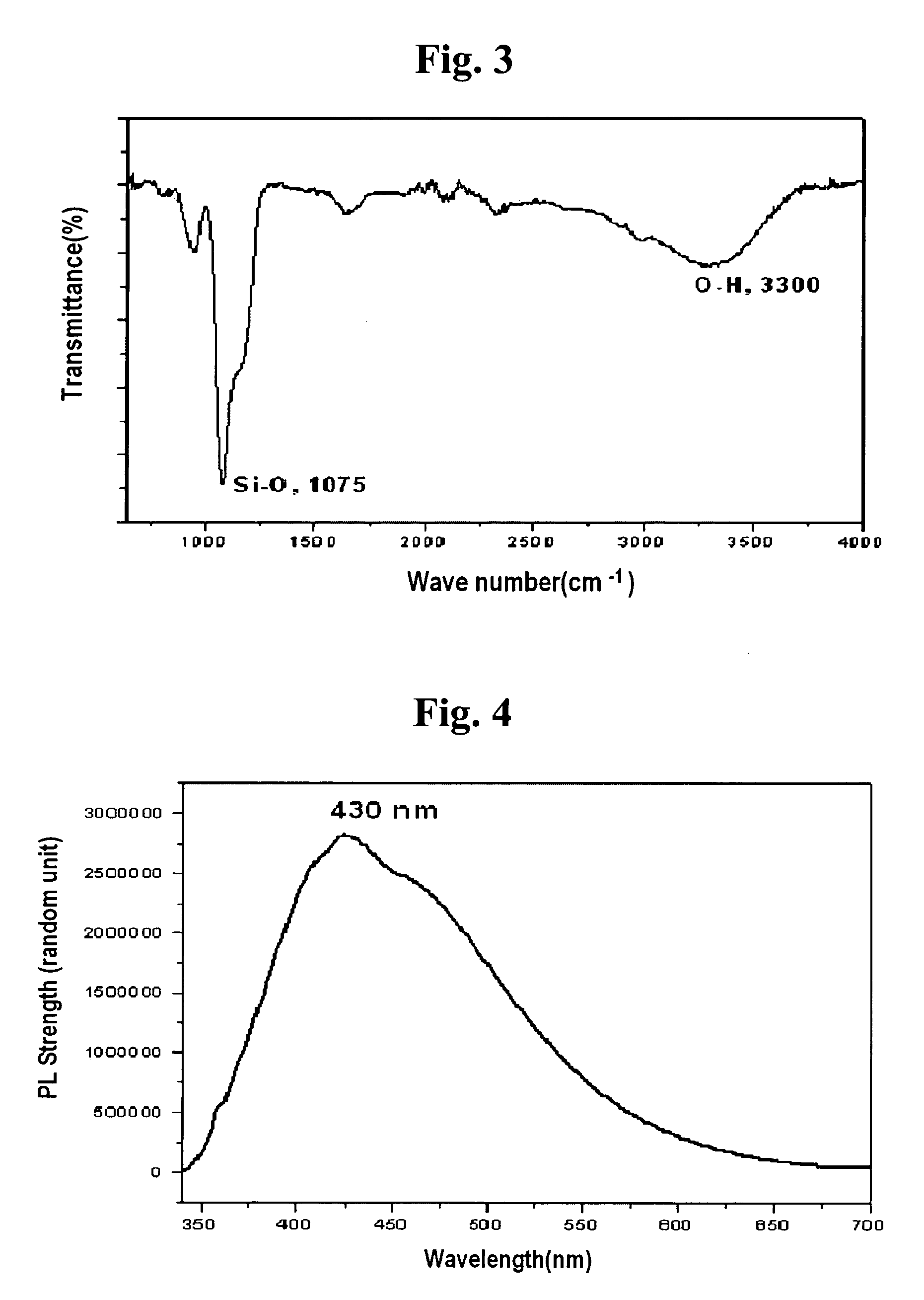
Method of preparing biocompatible silicon nanoparticles
InactiveUS20070098988A1Easy to mass produceGood biocompatibilityMaterial nanotechnologySurgical adhesivesDispersion stabilityDiethylene glycol diethyl ether
There is provided a method of preparing biocompatible silicon nanoparticles, which comprises the steps of forming a silicon nanoparticle colloid by ultrasonic treatment of Si-containing Zintl salt in diethylene glycol diethyl ether (DGDE), and introducing hydroxyl groups into the surface of the silicon nanoparticle by treating the silicon nanoparticle colloid with a halogenated hydrogen solution. The method of the present invention can easily mass-produce silicon nanoparticles having high dispersion stability in an aqueous solution and biocompatibility in a high yield.
Owner:KOREA INST OF SCI & TECH
Aligning agent for liquid crystal and liquid-crystal alignment film obtained with the same
ActiveUS7537812B2Good film uniformityLiquid crystal compositionsThin material handlingCrystallographyDiethylene glycol diethyl ether
To provide a liquid crystal aligning agent which gives a coating film with good uniformity regardless of drying temperature after coating, and a liquid crystal alignment film having good coating film uniformity.A liquid crystal aligning agent comprising at least one polymer selected from a polyamic acid and a soluble polyimide, diethylene glycol diethyl ether, and dipropylene glycol monomethyl ether, and a liquid crystal alignment film obtained by printing this liquid crystal aligning agent by a flexographic printing method.
Owner:NISSAN CHEM CORP
Method for preparing asphalt cleaners capable of effectively removing residual asphalt
InactiveCN106047537AEfficient removalEasy to cleanInorganic/elemental detergent compounding agentsOrganic detergent compounding agentsDiethylene glycol diethyl etherCOCONUT ACID
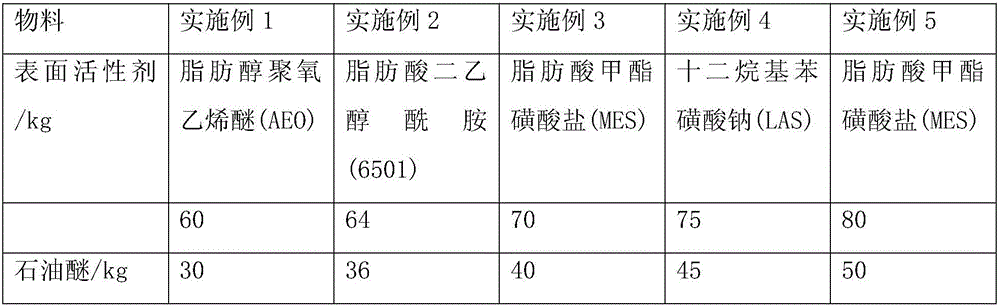
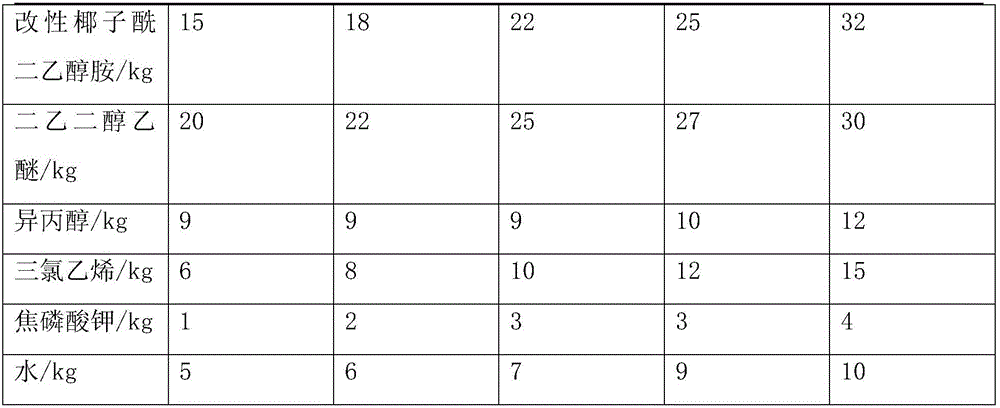
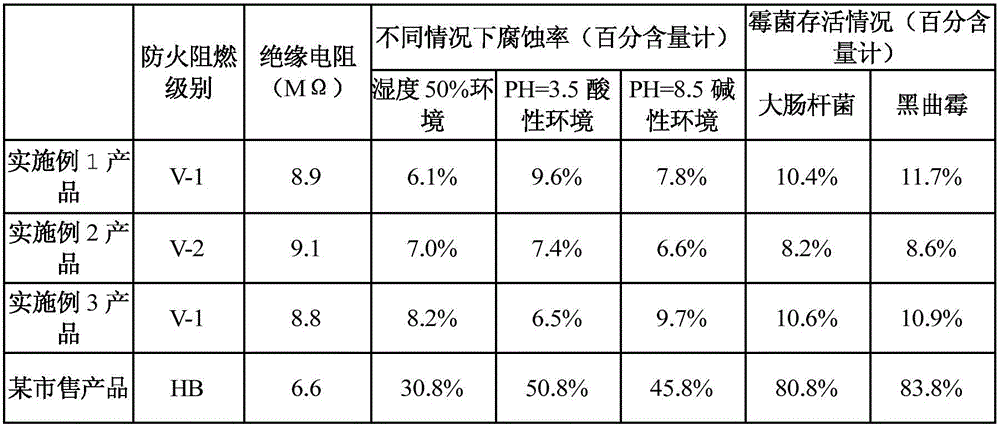
The invention provides a method for preparing asphalt cleaners capable of effectively removing residual asphalt. The method includes adding, by weight, 5-10 parts of water into a container at first, then sequentially adding, by weight, 60-80 parts of surfactants and 30-50 parts of petroleum ether into the container and stirring the water, the surfactants and the petroleum ether for 3-5 min to obtain preliminary mixed liquid; sequentially adding, by weight, 15-32 parts of modified coconut acid diethanolamide and 9-12 parts of isopropyl alcohol into the preliminary mixed liquid and mechanically stirring the modified coconut acid diethanolamide, the isopropyl alcohol and the preliminary mixed liquid for 10-20 min to obtain mixed liquid; adding, by weight, 20-30 parts of diethylene glycol diethyl ether into the mixed liquid, uniformly mixing the diethylene glycol diethyl ether and the mixed liquid with each other, then sequentially adding, by weight, 6-15 parts of trichlorethene and 1-4 parts of potassium pyrophosphate into the mixed liquid and manually stirring the diethylene glycol diethyl ether, the mixed liquid, the trichlorethene and the potassium pyrophosphate to obtain the asphalt cleaners. The method has the advantages that good cleaning effects can be realized by the asphalt cleaners, the asphalt cleaners are high in utilization rate, low in cost and little in environmental pollution, and processes for preparing the asphalt cleaners are simple.
Owner:TIANJIN KANGYUAN ENG MACHINERY
Flame retardation and antibiosis fabric
InactiveCN106009857AExcellent flame retardantGood acid and alkali resistanceFireproof paintsAntifouling/underwater paintsDiethylene glycol diethyl etherPolymer science
The invention relates to a flame retardation and antibiosis fabric. A gunny cloth fabric is bonded with a protection coating layer, and the coating layer comprises a-olefin elastomer, polymethyl methacrylate, isoprene rubber, tall oil fatty acid, butyl acetate, allyl sulfide, diethylene glycol diethyl ether, diethylene glycol butyl ether, propiconazole, sodium monofluorophosphate, dodecyl ethoxy sulfobetaine, dimethyl fumarate, cuprous chloride, metatitanic acid, silicon carbide fibers, phenyltriethoxysilane, chromium oxide green, chromium chloride, barium chromate powder, sodium cellulosate, trimethyl phosphate, polyacrylonitrile fibers, dibasic lead stearate, tris(butoxyethyl)phosphate, starch ether, vinyltris(beta-methoxyethoxy)silane, ethylamine, diethylenetriamine, alkylphenol polyoxyethylene and divinyl benzene. The flame retardation and antibiosis fabric has superior flame, acid-alkali, mildew and bacterium resistance, and improved performances.
Owner:李红玉
Preparation method of soluble low-temperature rapid imidization polyimide film
The invention discloses a preparation method of a soluble and low-temperature rapid imidization polyimide film, and belongs to the technical field of polyimide film preparation. The method comprises the following steps: a polar aprotic organic solvent with a boiling point lower than 200 DEG C is adopted as a solvent, and the polar aprotic organic solvent comprises one or more of diethylene glycol dimethyl ether, diethylene glycol diethyl ether, N,N-dimethyl acetamide, N,N-dimethyl formamide and dimethyl sulfoxide. According to the present invention, the polyimide film can be rapidly prepared through the imidization at the temperature of less than 200 DEG C, and the imidization process does not require the use of any catalyst, such that the important practical significance is provided for the production efficiency improving and the energy consumption reducing.
Owner:富优特(山东)新材料科技有限公司
Viscosity breaking agent for oil well produced liquid containing polymer or gel
The invention relates to a viscosity breaking agent for an oil well produced liquid containing polymer or gel. The viscosity breaking agent comprises the following components in percentage by weight: 12.15-13.05% of polyoxyethylene polyoxypropylene propylene glycol ether, 13.45-14.15% of fatty alcohol-polyoxyethylene ether phosphate monoester ammonium salt, 14.5-15.0% of fatty alcohol-polyoxyethylene ether phosphate monoester, 0.01-0.015% of alpha-carotene, 1.05-1.35% of N,N'-butylidene bilauroyl ammonium bromide, 2.35-3.0% of diethylene glycol diethyl ether, 0.5-0.65% of sodium hydroxide, 0.001-0.0015% of fluorocarbon surfactant FN-2, 0.45-0.5% of sodium phosphate and the balance of water. The invention has the advantage that when the addition quantity of the viscosity breaking agent is 500-800 mg / l, the polymer or gel is dispersed into nonaggregated small particles to be exploited out of ground, and the load of oil well pumping units is reduced. After being tested in more than 10 oil wells, the load of the oil well pumping units is reduced by 81.26% on average.
Owner:PETROCHINA CO LTD
Composition for forming a liquid crystal alignment film, and liquid crystal display device
ActiveUS8057868B2Good coating performanceSuppress display unevennessLiquid crystal compositionsPlastic/resin/waxes insulatorsDiethylene glycol diethyl etherLiquid-crystal display
The present invention provides: a composition for forming a liquid crystal alignment film capable of forming a liquid crystal alignment film excellent in evenness; and a liquid crystal display device. The present invention provides a composition for forming a liquid crystal alignment film, wherein the composition comprises: a material for forming a liquid crystal alignment film; diethylene glycol diethyl ether; diisobutyl ketone; and at least one of γ-butyrolactone and N-methyl-2-pyrrolidone as solvents.
Owner:SHARP KK +1
Non-aqueous inkjet ink and ink set
Owner:GENERAL CO LTD
Light sensitive resin composition
ActiveCN1702554AHigh light sensitivityImprove transmittanceStatic indicating devicesPhotosensitive materials for photomechanical apparatusDiethylene glycol diethyl etherCarboxylic acid
The photosensitive resin composition comprises (a) an acrylic copolymer obtained by copolymerizing (i) an unsaturated carboxylic acid, an unsaturated carboxylic acid anhydride or a mixture of them, (ii) an epoxy-containing unsaturated compound and (iii) an olefinically unsaturated compound, (b) a 1,2-quinonediazido compound and (c) one or more solvents selected from the group consisting of benzyl alcohol, hexyl alcohol, diethylene glycol dimethyl ether, diethylene glycol methylethyl ether, diethylene glycol diethyl ether, dipropylene glycol diethyl ether, dipropylene glycol dimethyl ether and dipropylene glycol methylethyl ether. So this invention provides a photosensitive resin composition which is excellent in performances such as sensitivity transmittance, insulation and chemical resistance, remarkably improves flatness and coating property in particular, and is suitable for forming an interlayer insulating film in a process for fabricating LCD, etc.
Owner:DONGJIN SEMICHEM CO LTD
Curable composition
ActiveUS20110253426A1Increase flexibilityExcellent in long-term electrical insulation propertyPolyurea/polyurethane coatingsConductive pattern formationElectricityDiethylene glycol diethyl ether
It is an object of the present invention to provide a curable composition which provides a cured product excellent in low warpage properties and long-term electrical insulation reliability and causes little bleeding during screen printing. The present invention is a curable composition comprising the following components (a) to (e): a component (a): polyurethane having a functional group capable of curing reaction and a carbonate bond, a component (b): γ-butyrolactone, a component (c): diethylene glycol diethyl ether, a component (d): inorganic fine particles and / or organic fine particles, and a component (e): a compound having two or more epoxy groups in one molecule.
Owner:NIPPON POLYTECH CORP
Viscosity reducer for gathering and transferring of single pipes and preparation method thereof
ActiveCN102382635AReduce back pressureDrilling compositionDiethylene glycol diethyl etherAlkylphenol
The invention discloses a viscosity reducer for gathering and transferring of single pipes, which is applied to reducing the wellhead back pressure in the gathering and transferring processes of crude oil single pipes. The viscosity reducer consists of the following components in percentage by weight: 3.5-4.5 percent of nonylphenol polyoxyethylene ether sodium carboxymethyl, 0.5-0.65 percent of liquid guanidine hydrochloride, 6.8-8.2 percent of polyoxyethylene alkylphenol sodium sulfate, 0.5-0.55 percent of sodium pyrophosphate, 12.5-14.5 percent of diethylene glycol diethyl ether, 5.5-6.5 percent of pre-film drag reduction viscosity reducer BN-99, 0.005-0.006 percent of arginine and the balance of water, wherein the sum of the percentage by weight of each component is 100 percent. The viscosity reducer has the effect that: when 150-200 mg / l of viscosity reducer is added, the wellhead back pressure of an oil well can be lowered by 25-30 percent.
Owner:PETROCHINA CO LTD
Lustrous electromagnetic wave transmissive coating film, electromagnetic wave transmissive coating material composition for forming this film, and method of forming electromagnetic wave transmissive coating film therewith
InactiveUS20130156972A1BalanceHighly lustrous appearancePretreated surfacesInksDiethylene glycol diethyl etherDiethyl ether
A lustrous electromagnetic wave transmissive coating film includes: metal nano-particles containing one or more kinds of metals; and a first resin containing an oxazoline group and, a second resin containing a carboxyl group, in the resin component the carboxyl group derived from the second resin being present in a molar ratio of 0.03 to 50 times the oxazoline group derived from the first resin; wherein the resin component is soluble in ethanol, or, when water is added to a diethylene glycol diethyl ether solution obtained by dissolving 0.5 g of the resin component in 10 ml of diethylene glycol diethyl ether, an addition amount of the water until the diethylene glycol diethyl ether solution becomes turbid is 1.5 ml or more.
Owner:TOYOTA JIDOSHA KK
Conductive silver paste and preparation method thereof
InactiveCN108735337AShorten the production cycleIncrease productivityNon-conductive material with dispersed conductive materialCable/conductor manufactureDiethylene glycol diethyl etherSilver paste
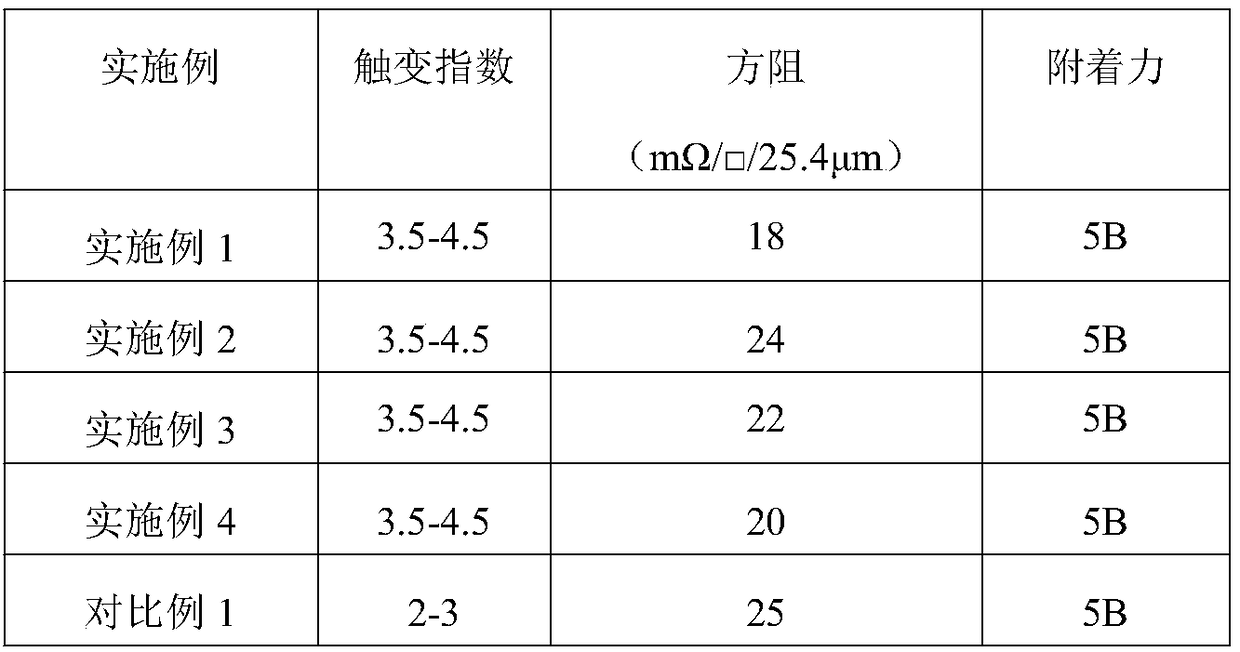
The invention discloses a conductive silver paste and a preparation method thereof, wherein the conductive silver paste comprises the following components and respective percentages: silver powder of45-80 percent, an organic carrier of 15-50 percent, an activated thixotropic agent of 0.6-3.5 percent, and an auxiliary agent of 0.4-2.0 percent; the organic carrier comprises the following componentsand respective percentages: an organic resin of 10-20 percent, and an organic solvent of 80-90 percent; and the organic solvent is selected from diethylene glycol, dibasic acid Ester (DBE), diethylene glycol diethyl ether acetate, and dibutyl phthalate. The invention combines the preparation of the organic binder phase and the organic carrier, which shortens the production cycle and improves theproduction efficiency. The invention is capable of avoiding the loss of activity due to improper dispersion of the thixotropic agent and increasing the thixotropic index of the conductive silver pasteby separately dispersing the thixotropic agent and the organic resin in the organic solvent, and capable of fully exerting the thixotropic properties by grinding the commercially available polyamidewax in a grinder to obtain polyamide wax powder having a smaller particle size.
Owner:湖南省国银新材料有限公司
Glass cleaning agent
InactiveCN104194973AStrong decontaminationLess corrosiveNon-ionic surface-active compoundsDetergent compounding agentsDiethylene glycol diethyl etherCleansing Agents
A glass cleaning agent comprises the following raw materials in percentage by mass: 70-80 parts of diethylene glycol diethyl ether, 20-25 parts of a green tea extract, 15-20 parts of succinate carbonate, 20-30 parts of marseille soap, 10-15 parts of ethanol and 10-20 parts of dodecyl glucoside. The preparation method of the green tea extract comprises the following steps: taking 15 parts of green tea, adding 600 parts of water, decocting for 1.2h, taking out decoction, and filtering to obtain the green tea extract. The glass cleaning agent is strong in detergency and low in corrosion.
Owner:遵义市斌灏信息咨询有限公司
Polytetrafluoroethylene product surface treatment agent and preparation method thereof
ActiveCN103351474AImprove working conditionsSolve the problem of safety productionDiethylene glycol diethyl etherAfter treatment
The invention discloses a polytetrafluoroethylene product surface treatment agent which comprises the following components in parts by weight: 50-280 parts of naphthalene, 10-50 parts of metallic sodium and 600-800 parts of a solvent, wherein the solvent is one of 1,4-dioxane, glycol dimethyl ether, diethylene glycol dimethyl ether, diethylene glycol diethyl ether, ethylene glycol butyl methyl ether, tetraethylene glycol dimethyl ether and diethylene glycol isobutyl ether. The invention further provides a preparation method for the polytetrafluoroethylene product surface treatment agent. The polytetrafluoroethylene product surface treatment agent has the advantages of safety, environmental pollution and high treatment capability; and a polytetrafluoroethylene product obtained after treatment of the surface treatment agent is good in quality and high in qualified rate.
Owner:ZHEJIANG GREEN NEW MATERIALS +1
Multi-resin composite wire enamel and preparation method thereof
InactiveCN103725148AExcellent adhesionReduce pinholesPolyamide coatingsPolyesterDiethylene glycol diethyl ether
The invention discloses multi-resin composite wire enamel and a preparation method thereof. The multi-resin composite wire enamel is characterized by being prepared from raw materials in parts by weight as follows: 12-15 parts of phenolic resin, 3-4 parts of polyester silicone oil, 2-3 parts of polyvinyl chloride, 2-3 parts of isocyanate, 7-9 parts of diethylene glycol diethyl ether, 5-7 parts of dipropylene glycol monomethyl ether, 12-15 parts of polyisobutene, 3-8 parts of ABS (acrylonitrile butadiene styrene) resin, 12-13 parts of PP (polypropylene) resin, 12-15 parts of nylon 12, 7-9 parts of an assistant, 100-110 parts of m, p-cresol and 100-110 parts of xylene. The production cost of an enameled wire is low, the production speed is high, and the labor productivity is improved.
Owner:铜陵天河特种电磁线有限公司
Bridge connection rail surface environmental protection coating for civil and architectural engineeringroad and bridge construction
InactiveCN106084940AExcellent flame retardantGood acid and alkali resistanceFireproof paintsAntifouling/underwater paintsDiethylene glycol diethyl etherMaterials science
The invention relates to a bridge connection rail surface environmental protection coating for civil and architectural engineeringroad and bridge construction. The bridge connection rail surface environmental protection coating comprises the following components: PVC resin powder, butadiene-styrene rubber, hexamethylenediisocyanate, propargyl chloride, isothiazolinone, glass powder, soda ash, lead iodide powder, chrome green, zinc chromate, magnesium silicate powder, magnesium methacrylate, sodium polymethacrylate, spodumene powder, zinc sulfide powder, asbestos powder, nut oil, methyl acetate, soya oil acid, diethylene glycol diethyl ether, isobutyl acrylate, tripropylene glycol diacrylate, dibasyl lead sulfate, sodium diethylhexyl sulfosuccinate, epoxy silane crosslinker, sodium dioctylsulfosuccinate, sorbitol, sodium dodecyl sulfonate, alkyl diphenyl phosphate, and ethoxylated lauryl alcohol sulfate. The product provided by the invention has superior flame-retardant, acid and alkali-proof, mildew-proof and antibacterial properties, and the product properties are improved.
Owner:梁方英
Ink composition and preparation method thereof
InactiveCN104356760AReliable electrical connectionModerate viscosityInksDiethylene glycol diethyl etherEthylene glycol
The invention discloses an ink composition and a preparation method thereof. The ink composition is prepared from the following components in parts by weight: 15-25 parts of a metallic pigment, 3.5-5 parts of tween, 3-5 parts of diglycidyl phthalate, 2.5-3 parts of polyepoxysuccinic acid, 2-4 parts of white carbon black, 2-3 parts of azodiisobutyronitrile, 2-3 parts of urea, and 20-30 parts of diethylene glycol diethyl ether. The invention also provides a preparation method of the ink composition. The preparation method comprises the following steps: (1) weighting 15-25 parts of the metallic pigment, 3.5-5 parts of tween, 3-5 parts of diglycidyl phthalate, 2.5-3 parts of polyepoxysuccinic acid, 2-4 parts of white carbon black, 2-3 parts of azodiisobutyronitrile and 2-3 parts of urea; (2) adding the weighted components into a three-roll grinder to grind for 10-20 minutes at a temperature of 110-125 DEG C, and carrying out heat preservation for 2-3 hours at a temperature of 155-160 DEG C; and (3) adding 20-30 parts of diethylene glycol diethyl ether into a product obtained in the step (2), and carrying out uniform ultrasonic mixing, so that the ink composition is obtained.
Owner:苏州冰心文化用品有限公司
High-performance printing ink cleaning agent
InactiveCN105385220AImprove decontamination abilityEasy to cleanChemical paints/ink removersDiethylene glycol diethyl etherActive agent
A high-performance printing ink cleaning agent is prepared from, by weight, 4-7 parts of dehydrated sorbitol fatty acid ester, 3-5.2 parts of isopropanol, 2-4.6 parts of surfactant, 5-8 parts of sodium dodecyl benzene sulfonate, 2-4 parts of fatty alcohol-polyoxyethylene ether, 1.2-2.4 parts of dibutyl phthalate, 0.1-0.2 part of sodium hydroxide, 3-4 parts of benzotriazole, 3.2-4.6 parts of diethylene glycol diethyl ether, 6-8 parts of D-limonene, 0.7-1.3 parts of hydroxy phosphonic acids, 1-2 parts of sodium silicate, 4.2-5.8 parts of molybdate, 4-6 parts of instant modified sodium disilicate and 1.2-3.5 parts of penetrating agent. The high-performance printing ink cleaning agent has the advantages of being high in dirt-removing power to printing ink, good in cleaning effect, high in dirt-removing power, high in safety, efficiency and performance, safe and free of pollution.
Owner:王丽萍
Preparation method for weak-base arylamine diazonium salt and method for preparing tertiary amine weak-base arylamine azo dye by weak-base arylamine diazonium salt
ActiveCN104926685AImprove protectionNo explosion hazardMonoazo dyesOrganic chemistryDiethylene glycol diethyl etherEthyl acetate
The invention relates to a preparation method for weak-base arylamine diazonium salt and a method for preparing tertiary amine weak-base arylamine azo dye by the weak-base arylamine diazonium salt, and belongs to the technical field of clean production of the dye. The preparation method comprises the following steps of adding naphthalenesulfonic acid and alkyl nitrite to weak-base arylamine solution in sequence, reacting for 20min to 60min at 0 DEG C to 25 DEG C, and performing solid-liquid separation to obtain weak-base arylamine diazonium salt solid; solvent for dissolving weak-base arylamine is tetrahydrofuran, 1,4-dioxane, glycol dimethyl ether, ethylene glycol diethyl ether, diethylene glycol dimethyl ether, diethylene glycol diethyl ether or ethyl acetate. The preparation method for the weak-base arylamine diazonium salt and the method for preparing the tertiary amine weak-base arylamine azo dye by the weak-base arylamine diazonium salt, disclosed by the invention, have the beneficial effects that no sulfuric acid is used in a process of preparing the weak-base arylamine diazonium salt and the tertiary amine weak-base arylamine azo dye, therefore the environment is protected.
Owner:DALIAN UNIV OF TECH
Environment-friendly type corrosion resistant composition for normal temperature descaling of metal materials
InactiveCN109628939ANo pollution in the processStrong adhesionDiethylene glycol diethyl etherHazardous substance
The invention discloses an environment-friendly type corrosion resistant composition for normal temperature descaling of metal materials. The environment-friendly type corrosion resistant compositioncan rapidly remove rust on the surfaces of the metal materials, and forms dual-layer protecting films on the surfaces of the metal materials to prevent secondary rusting. The environment-friendly typecorrosion resistant composition is prepared from the following components in percentage by mass: 12-20% of an acid agent, 3-8% of a reducing agent, 1-5% of an accelerant, 3-8% of a penetrating agent,1-2% of a non-ionic surface active agent, and the balance of pure water, wherein the acid agent is an unsaturated organic acid and chemical compound thereof; and the penetrating agent is prepared from the following components by molar ratio: 2-4 of dipropylene glycol, 2-4 of diethylene glycol diethyl ether, and 3-5 of diethylene glycol butyl ether. The environment-friendly type corrosion resistant composition is a synthetical composition capable of simultaneously oil removing, descaling and anti-rust-passivating, contains no inorganic phosphorus or organic phosphorus and other toxic heavy metals, in the treatment process, needs no heating, does not generate acid mist and harmful substances and cannot pollute the environment, and has excellent adhesive force with subsequent coatings.
Owner:GUANGZHOU HONGSHUO ENVIRONMENTAL TECH
Preparation method for 9,9-bis[6-(2-hydroxyethoxy)naphthyl]fluorene with high purity and high bulk density
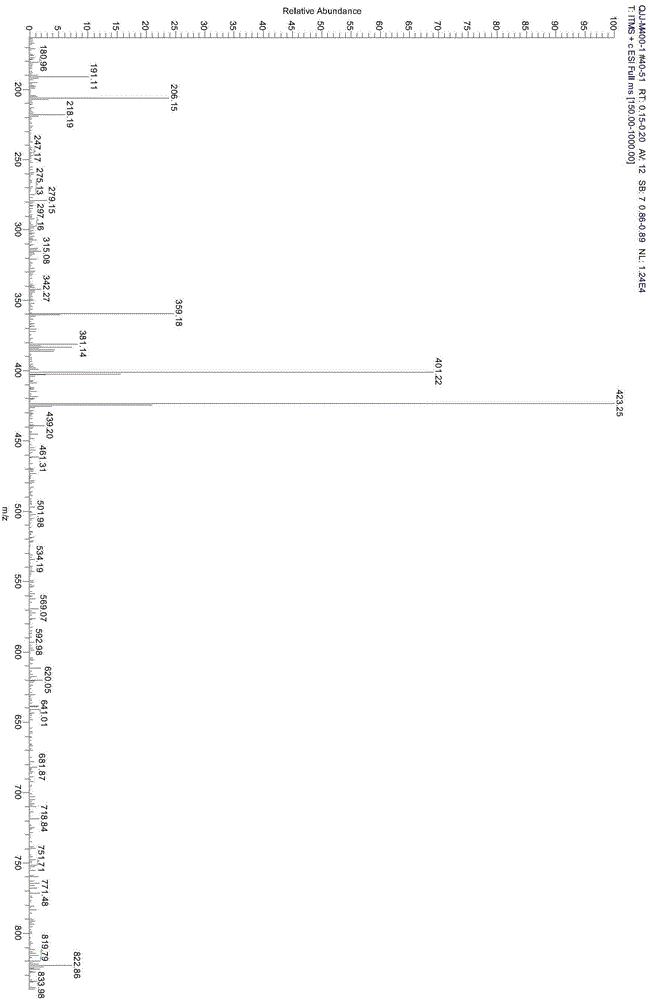
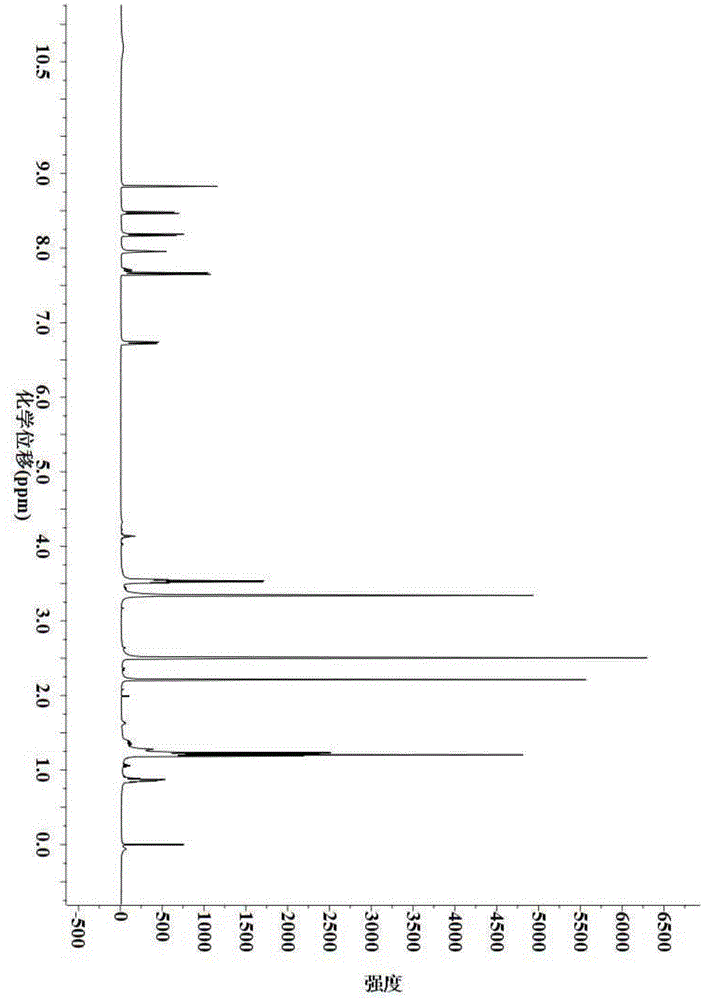
ActiveCN108017521AInhibition formationShort reaction timeEther preparation by ester reactionsDiethylene glycol diethyl etherN dimethylformamide
The invention provides a preparation method for 9,9-bis[6-(2-hydroxyethoxy)naphthyl]fluorene with high purity and high bulk density. The 9,9-bis[6-(2-hydroxyethoxy)naphthyl]fluorene with high purity and high bulk density is prepared by condensation of 9,9-bis(2-hydroxylnaphthyl)fluorene and ethylene carbonate under an alkaline condition with polar non-protonic solvents like N,N-dimethylacetamide,N,N-dimethylformamide, N-methylpyrrolidone, dimethylsulfoxide, diethylene glycol dimethyl ether, diethylene glycol diethyl ether, triethylene glycol dimethyl ether, triethylene glycol diethyl ether and polyethylene glycol dimethyl (or diethyl) ether as reaction accelerators. The 9,9-bis[6-(2-hydroxyethoxy)naphthyl]fluorene has a purity (HPLC) of larger than 99.0%, a bulk density of larger than 0.4g / cubic centimeter, a loss on drying of less than 0.5% (at 120 DEG C, 30 min), and a melt absorption peak at 213 to 222 DEG C. The 9,9-bis[6-(2-hydroxyethoxy)naphthyl]fluorene has a powder X-ray diffraction peak (XRPD) spectrum which has a morphology as shown in a figure 3 of the invention and has characteristic diffraction peaks with 2theta values of 7.7 + / - 0.20, 8.8 + / - 0.20, 12.5 + / - 0.20, 14.3 + / - 0.20, 15.5 + / - 0.20, 17.7 + / - 0.20, 18.7 + / - 0.20, 19.5 + / - 0.20, 20.4 + / - 0.20, 20.9 + / - 0.20, 21.2 + / - 0.20, 22.2 + / - 0.20, 22.7 + / - 0.20, 23.1 + / - 0.20, 24.8 + / - 0.20, 25.4 + / - 0.20, 25.9 + / - 0.20 and 26.7 + / - 0.20.
Owner:JIANGSU EVER GALAXY CHEM CO LTD
Kitchen ventilator cleaning agent and preparation method thereof
InactiveCN106978263AEasy to removeNot corrosiveInorganic/elemental detergent compounding agentsOrganic detergent compounding agentsDiethylene glycol diethyl etherPreservative
The invention discloses a kitchen ventilator cleaning agent and a preparation method. The preparation method comprises the following steps: weighing the following components in parts by mass: 15-20 parts of an emulsifying agent, 8-10 parts of a surfactant, 5-10 parts of ethanol, 3-5 parts of diethylene glycol diethyl ether, 2-4 parts of triethanolamine, 2-4 parts of essence, 1-3 parts of a preservative, 1-3 parts of thickening powder and 100-120 parts of water; dissolving the emulsifying agent and the surfactant in the water, and uniformly stirring for subsequent use; mixing the ethanol, the diethylene glycol diethyl ether and triethanolamine, and uniformly stirring for subsequent use; mixing the products, stirring uniformly, and standing for 10-20 minutes; and then adding the essence, the preservative and the thickening powder while stirring, and stirring uniformly to obtain the finished product. The prepared cleaning agent is convenient to use, gentle in action, low in cost, high in oil contamination removal ability, high in efficiency, safe and pollution-free, and gentle in smell.
Owner:侯淑璐
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
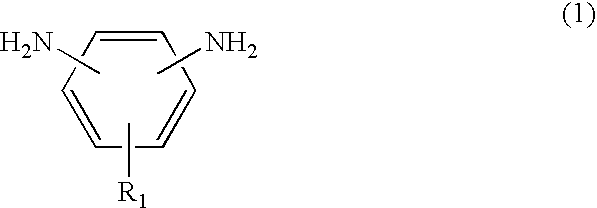




![Preparation method for 9,9-bis[6-(2-hydroxyethoxy)naphthyl]fluorene with high purity and high bulk density Preparation method for 9,9-bis[6-(2-hydroxyethoxy)naphthyl]fluorene with high purity and high bulk density](https://images-eureka.patsnap.com/patent_img/6ee47f45-1f3f-4c09-b21a-5cad54fbdc51/171027173654.png)
![Preparation method for 9,9-bis[6-(2-hydroxyethoxy)naphthyl]fluorene with high purity and high bulk density Preparation method for 9,9-bis[6-(2-hydroxyethoxy)naphthyl]fluorene with high purity and high bulk density](https://images-eureka.patsnap.com/patent_img/6ee47f45-1f3f-4c09-b21a-5cad54fbdc51/171027173658.png)
![Preparation method for 9,9-bis[6-(2-hydroxyethoxy)naphthyl]fluorene with high purity and high bulk density Preparation method for 9,9-bis[6-(2-hydroxyethoxy)naphthyl]fluorene with high purity and high bulk density](https://images-eureka.patsnap.com/patent_img/6ee47f45-1f3f-4c09-b21a-5cad54fbdc51/171027173703.png)